Factors Influencing Yields in Extraction, Part II: Examining the Impact of Material Preparation
Part II of this series explores the impact of how material is prepared prior to extraction.
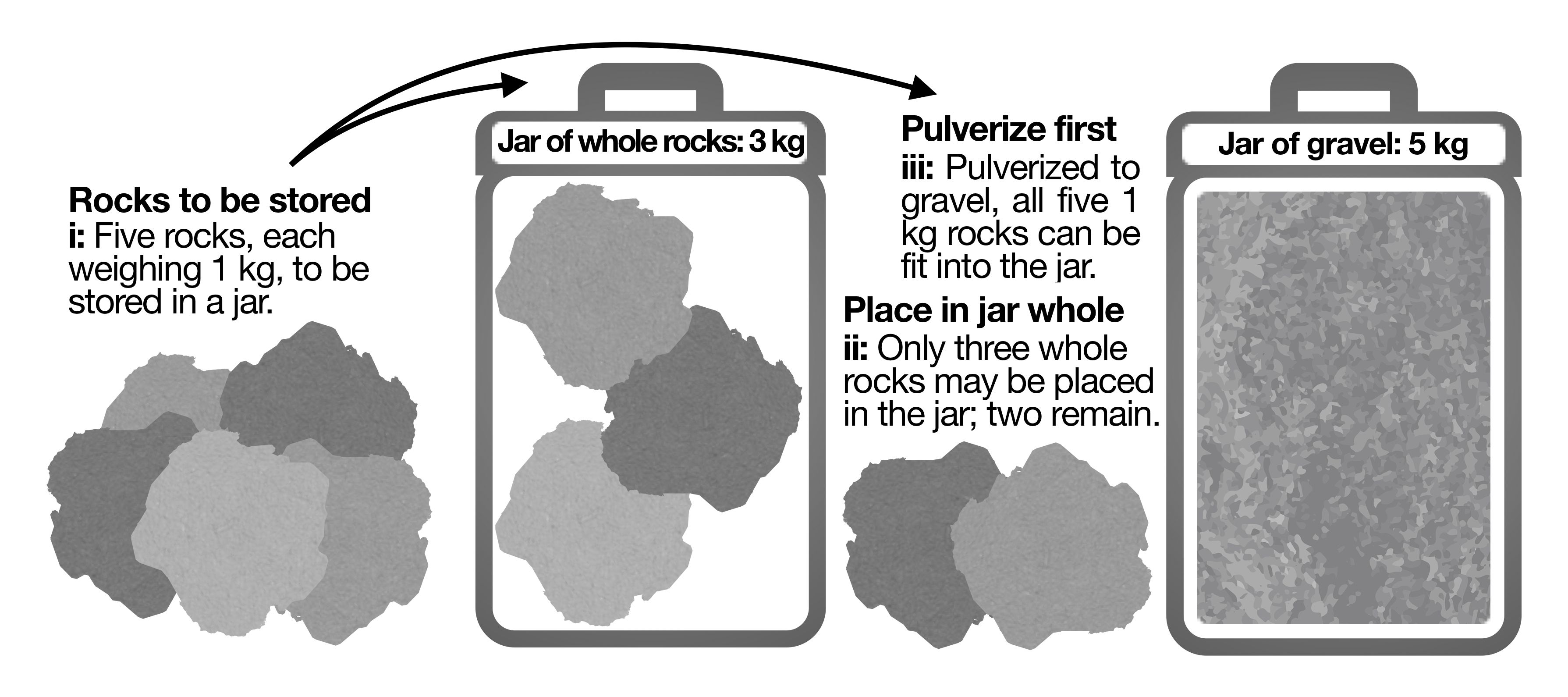
(Click to enlarge): Figure 1: Only a few large rocks can be maneuvered into a space of a given size. If the rocks are first pulverized, many more rocks’ worth of material can be fit into an identical space. Finer-sized material has better packing efficiency.
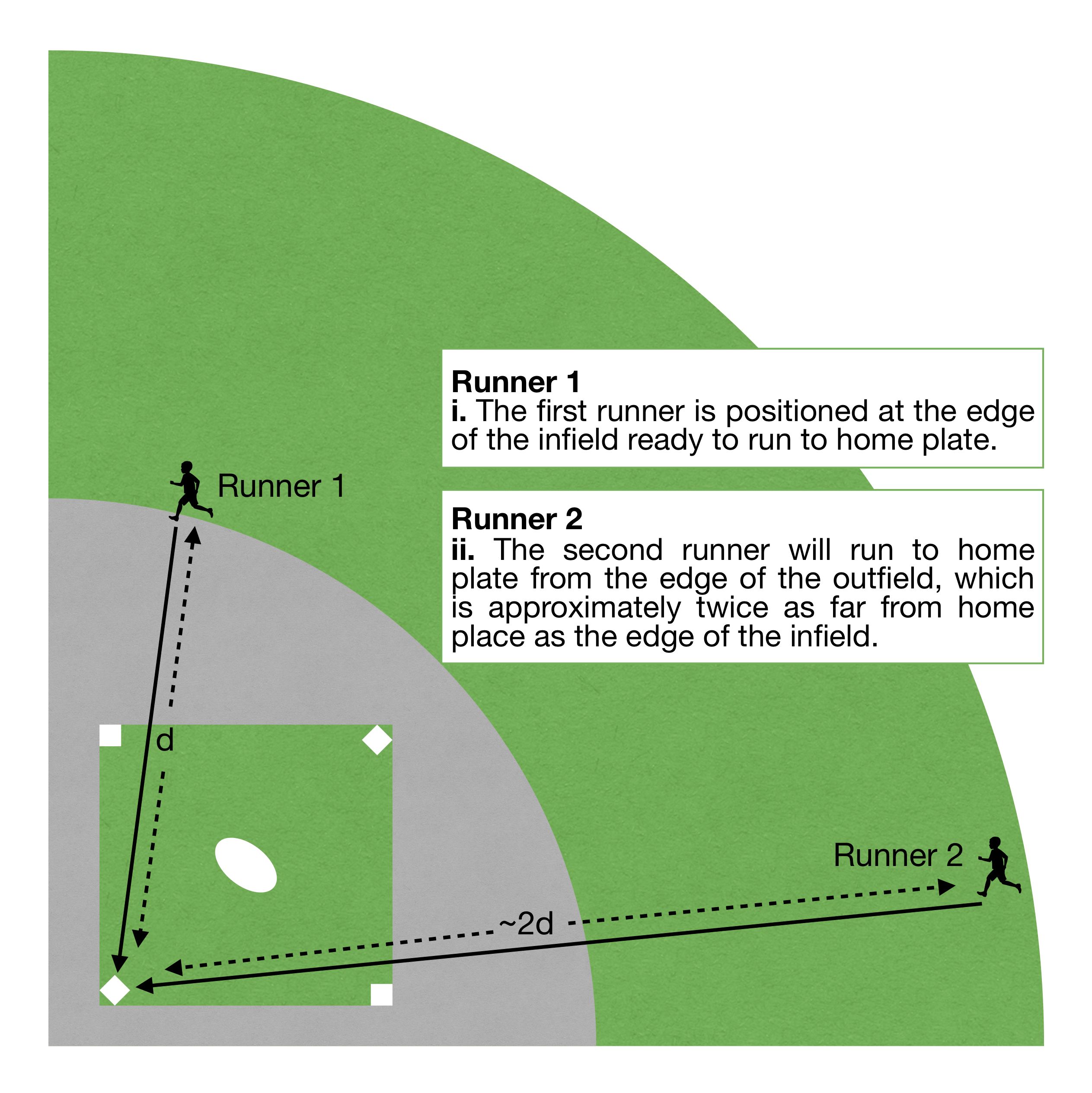
(Click to enlarge): Figure 2: A baseball diamond and two runners of identical speed who time a run to home plate. Runner 1 starts from the edge of the infield, Runner 2 from the edge of the outfield. Which athlete is expected to complete their run in the shortest amount of time?
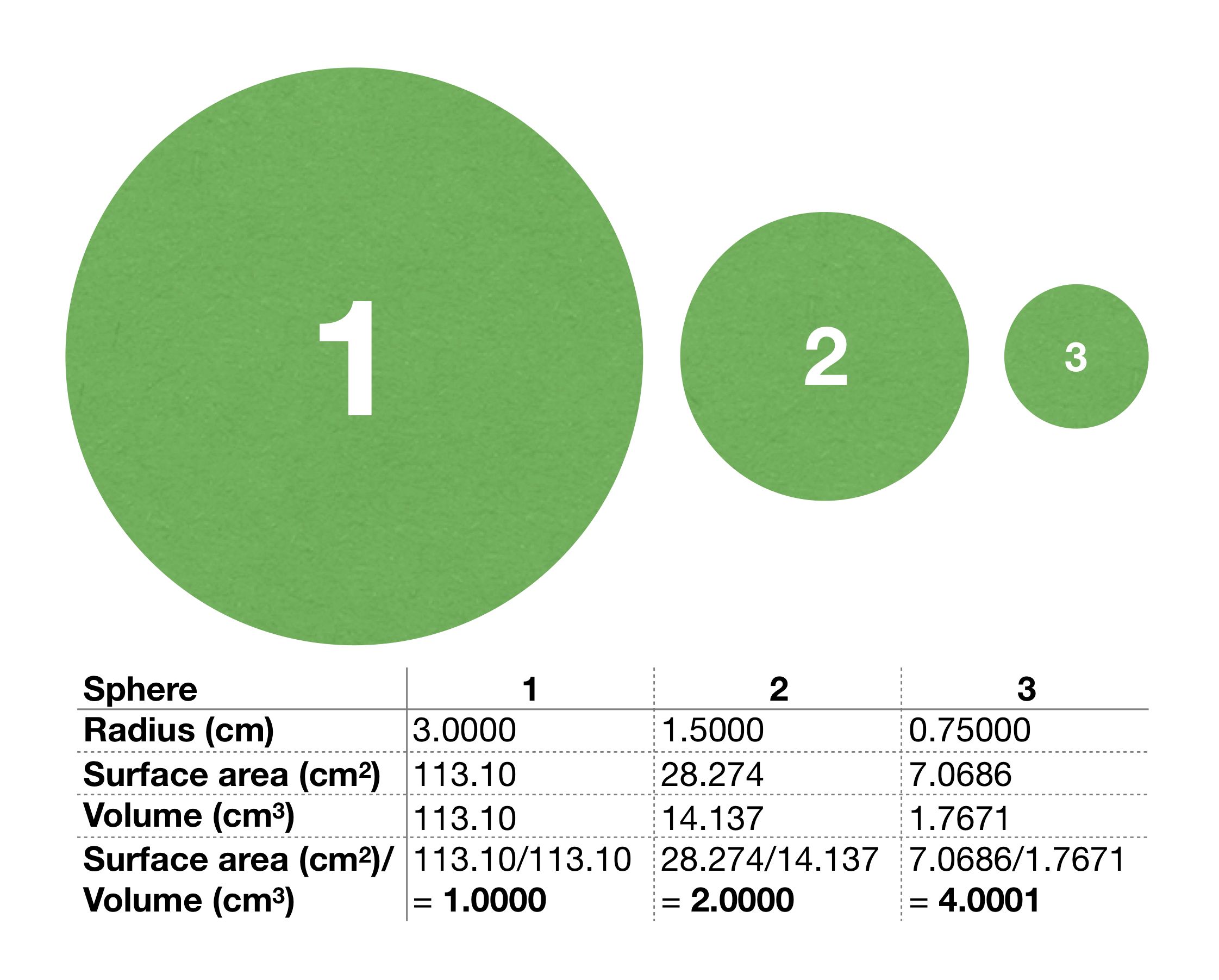
(Click to enlarge): Figure 3: A visual comparison of the physical attributes of three spheres, proxied by three circles. If one imagines hammering as many nails as possible into this set of spheres, the smallest would accommodate four times as many nails, compared to its volume, as the largest. A sphere of one quarter the radius has four times the surface area to volume ratio.
This three-part series began with Part I, an in-depth discussion of how the feedstock component makeup influences the yield obtained from an extraction. It examined starting material influences, including total mass of material loaded in the extraction chamber and the fraction of interest in the component makeup of the material. An overview of basic extraction mathematics with special focus on percent extraction efficiency calculations was also presented. Here, in Part II, we explore the impact of how material is prepared prior to extraction. To follow in the next issue, Part III will conclude the series with a discussion of the influence of total extraction time and processing parameters
In addition to the influence of how much material is committed to a given extraction, and what fraction of that material represents component(s) of interest, decisions regarding material preparation prior to commencing the extraction also play a role in the yield obtained.
Particle Size
Specifically, we consider the role of feedstock particle size and its effects on packing efficiency and solvent-matrix interactions.
Packing Efficiency
Intuitively, reducing the feedstock particle size prior to its addition to the extraction chamber maximizes the mass of feedstock that can ï¬t snugly into the space. In other words, in a ï¬xed-volume chamber, and given the same feedstock material, more feedstock will ï¬t into the volume of the chamber when ground to a ï¬ner size. One can equivalently consider trying to place rocks in a jar (Figure 1). Only a few large rocks can be maneuvered into a space of a given size. If the rocks are ï¬rst pulverized, however, many more rocks’ worth of material can be ï¬t into an identical space. Finer-sized material has better packing efï¬ciency.
Time to Reach the Center of a Sphere
Further, reducing the size of the feedstock particle reduces the distance that must be traversed by the solvent to penetrate the centre of the particle (feedstock particle radius). Figure 2 considers a baseball diamond and two runners of identical speed. The ï¬rst runner will start from the edge of the inï¬eld, the second from the edge of the outï¬eld; both time a run straight to home plate. For reference, home plate is approximately twice as far from the outï¬eld as it is from the inï¬eld. Which athlete is expected to complete their run in the shortest amount of time? For identical speeds of travel, a shorter travel time is required to traverse a shorter distance. In terms of extraction parameters, the solvent will reach the centre of a ï¬ner-ground feedstock particle more quickly than a coarser-ground one.
Surface-Area-to-Volume Ratio
Another factor for discussion with regards to particle size is the surface-area-to-volume ratio (SAV). Increasing the SAV increases interaction between the solvent and the feedstock matrix which, in turn, increases the rate at which the components of interest are brought into solution. This is easily the most complex of the effects to explain, as well as to internalize.
Figure 3 tabulates the radius, surface area, volume and SAV for each of three spheres, represented by three circles. As shown, a larger sphere has a larger radius, volume, and surface area. However, because these do not increase proportionally, the SAV actually decreases for larger spheres.
If one imagines hammering as many ï¬nishing nails as possible into a set of wooden spheres with dimensions as given in Figure 3, the smallest sphere would accommodate four times as many nails, compared to its volume, as the largest sphere. The largest sphere would still have more nails, but its volume is much less accessible.
Each point on the surface of the sphere acts as an avenue of interaction with the sphere’s volume. A smaller particle size provides more avenues of interaction per particle volume than does a larger particle size. This allows interaction of the solvent with the matrix of the feedstock material to occur more efï¬ciently.
Coextraction of Other Components
To round out the present discussion of the merits of reducing particle size, it is important to note one particular point regarding extraction without particle size reduction. The process of reducing particle size also disrupts plant cell structure. Rupturing cells provides increased access to the interior contents of the cell, some of which may not be desired in the end product. Thus, while the advantages of smaller particle size offer increased efï¬ciency in extraction, it can also mean more coextraction of other components. Extraction without particle size reduction can provide access to desirable components while employing the plant’s own structure to help minimize coextraction.
Trade-offs in total extraction time, extraction efï¬ciency and post-processing requirements thus have many layers of complexity as an operator prepares for an extraction. The choice of extraction parameters, discussed in the next part of this series, provides the operator with additional tools as they work to identify the most desirable pre-processing, extraction, and post-processing regime.
Conclusions
In summary, a smaller particle size provides two main efï¬ciency increases: an advantage in packing efï¬ciency, and an increased efï¬ciency of interaction of the solvent and feedstock matrix through reduced particle radius and improved surface-area-to-volume ratio. The process of reducing particle size may also increase coextraction of potentially undesirable components. These concepts, with those discussed in the series’ ï¬rst installment, conclude the discussion of starting-material inï¬uence on yields. Part III, the next and ï¬nal in the series, will examine the effects of processing parameters on an extraction’s output.
Krista Marie Kulczycki is a technical and scientific writer with Vitalis Extraction Technology in British Columbia, Canada. Aaron Godin is an application science manager with Vitalis Extraction Technology. Direct correspondence to: krista.kulczycki@vitalset.com.
How to Cite This Article:
Kulczycki K et al., Cannabis Science and Technology 2(3), 56-57 (2019)
