Hemp Regulations and the Restrictions on Innovation
Isolated CBD, full-spectrum oil, and even smokable hemp are all being diluted down by a draconian regulatory framework that needs to be changed to facilitate growth, innovation, and prosperity throughout the industry.
The current state of hemp regulations in the United States begs the question: why is the current hemp compliance threshold set to an arbitrary value of 0.3% w/w total tetrahydrocannabinol (THC)? This represents a lack of understanding of Group III cannabis/hemp genetics, which is causing farms, extractors, and ancillary businesses two- to three-times as much capital to get the same results in an oil or isolate product. This also impacts land use, nutrients application, and pesticide use; ultimately having deleterious effects on the environment. Our extensive research into hemp genetics has revealed that across the board a simple increase in cannabidiol (CBD) also causes a proportional increase in THC, irrespective of provenance. This increase does not affect the final potency of raw oils as extraction equalizes to the same ratio; however, the current compliance threshold of 0.3% total THC hinders the amount of oil that can be produced from a given acreage. As such, everyone in the industry is being punished by archaic laws that have little to no real-world application in the modern hemp markets. Farmers, breeders, and extractors should not be punished for creating and cultivating superior genetics. Isolated CBD, full-spectrum oil, and even smokable hemp are all being diluted down by a draconian regulatory framework that needs to be changed to facilitate growth, innovation, and prosperity throughout the industry.
For the better part of the last 10 years Cannabis Spp. varietals have been categorized as either Cannabis indica, Cannabis sativa, or Cannabis ruderalis, with the term hemp being more closely associated with the latter two sub-species. Nonetheless, the widespread hybridization that has resulted from systemic interbreeding of these varietals has led to a comparatively new classification system, whereby the relative expression of Delta-9-tetrahydrocannbinol (D9-THC) and cannabidiol (CBD) dictates a particular strain’s sub-type. For example, Group I cannabis refers to THC-dominant strains (>20:1 THC/CBD); Group II cannabis corresponds with ratioed genetics (~1:1 THC/CBD); and Group III cannabis (hemp) is comprised of CBD-dominant strains (>20:1 CBD/THC). Interestingly, Groups I–III cannabis can all express similar morphological structures (Figure 1), flavonoid and terpene profiles; with the primary differentiating factor being the final concentration of THC. Additionally, all three Groups can be observed coexisting together in putative wild landrace strains. This is to say even with the inbreeding of, for example, two Group II landrace plants for the production of “selfed” feminized seed that is homogeneous, the progeny will follow the principles of Mendelian inheritance, whereby 25% will be expressed as Group I cannabis, 50% will resemble Group II, and 25% will be representative of Group III cannabis (hemp). This particular genetic feature of cannabis and hemp presents a whole other issue plaguing the hemp industry; however, the topic of genetic stability of commercially-available hemp seed is a discussion for another day.

While the regulation of Groups I and II falls under the jurisdiction of cannabis; the regulation of Group III genetics is somewhat variable depending on the final THC content of the cured flower material. For example, prior to the recent changes to the United States Department of Agriculture’s (USDA) hemp guidelines in October 2019, the threshold for determining whether or not a plant was considered hemp was a D9-THC concentration of 0.3% weight/weight (w/w). However, the aforementioned changes maintained the value of 0.3% while now requiring a cumulative measure of total THC, which is comprised of the amount D9-THC plus the amount of its acidic precursor, tetrahydrocannabinolic acid (THCA), accounting for the loss of mass during decarboxylation (total THC = D9-THC + 0.877*THCA). This has left the American hemp industry in a highly restricted state, whereby most commercially-available genetics are no longer compliant and thus are considered THC-rich cannabis, which inherently requires different permitting and licensure.
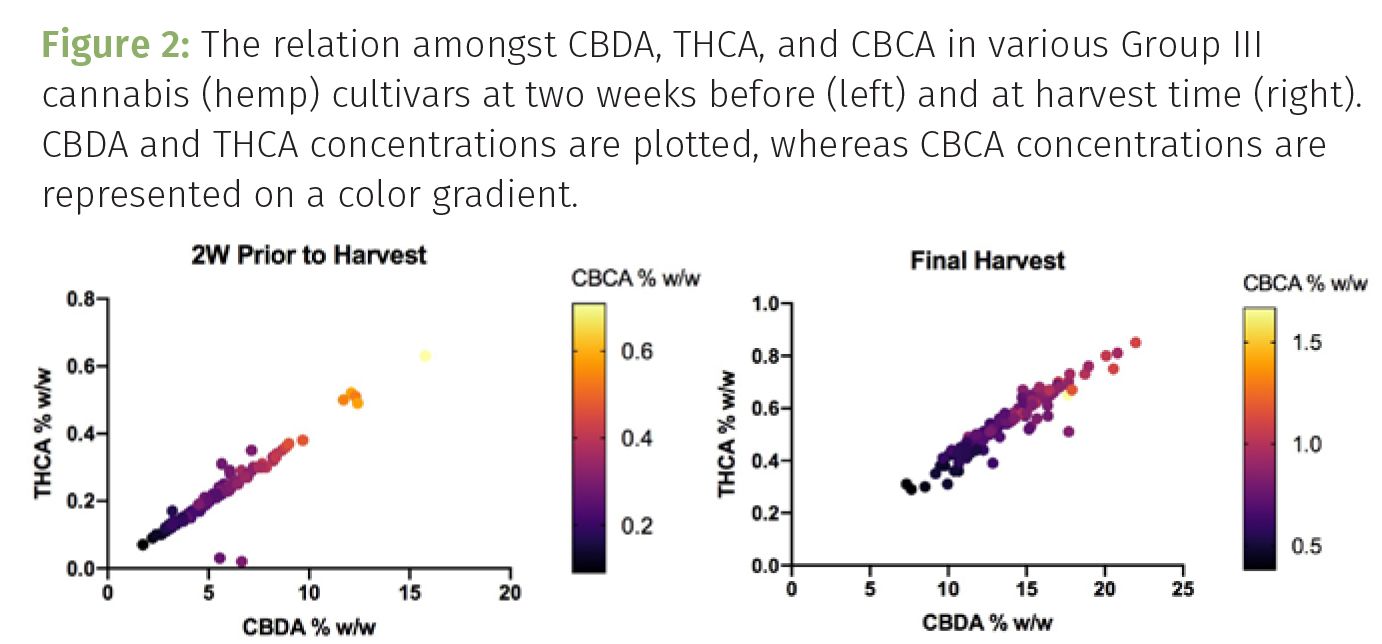
In fact, our own research indicates that because CBD and THC levels are linearly correlated in Group III cannabis, it is nearly impossible to produce compliant hemp material with a CBD concentration greater than 10% w/w (Figure 2). By comparison, the hemp regulations in select European markets such as Switzerland allow for up to 1.0% w/w total THC. This in turn presents Swiss cultivators with the opportunity to produce much more phytochemically dense flower material, with compliant genetics exceeding 25% w/w CBD in many cases. These more favorable regulations also allow for the enhanced expression of secondary cannabinoids such as cannabichromene (CBC) and cannabichromenic acid (CBCA), which demonstrates multicollinearity with CBD and THC (Figure 1). Additionally, the more accommodating regulations, whether they be 1.0% total THC or 0.3% D9-THC, allow for a greater expression of ancillary cannabinoid species such as cannabidivarin (CBDV) and cannabidivarinic acid (CBDVA), tetrahydrocannabivarin (THCV/THCVA), and cannabicyclol (CBL) and cannabicyclolic acid (CBLA) as these molecules are expressed in the same fragile, hair-like trichome structures that produce and house CBD and THC. As such, the more restricted hemp regulations (0.3% total THC) result in the production of less efficacious plant material, which is a tremendous disservice to both the cultivator and the end user.
Financial, Operational, and Environmental Implications
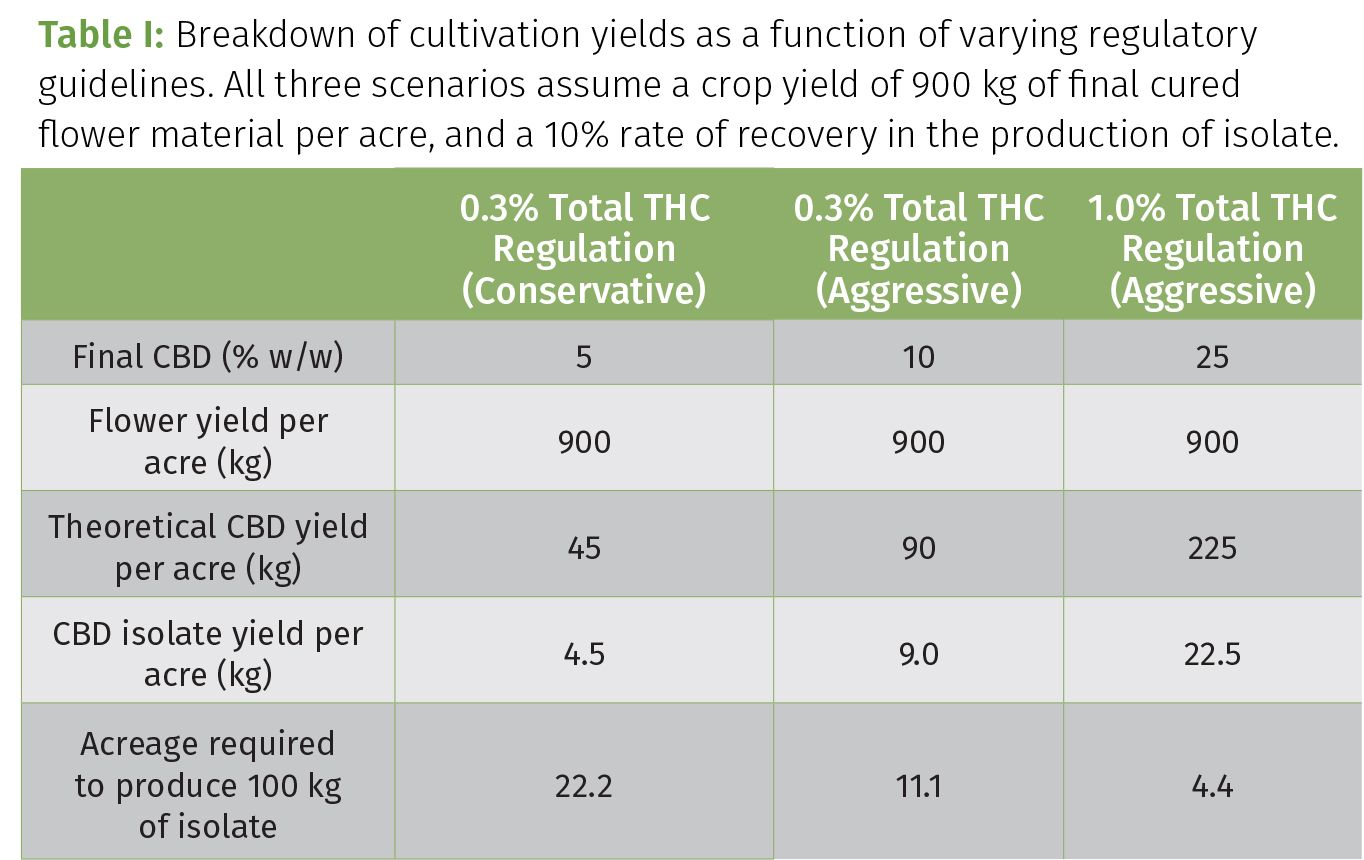
While the regulation-mediated reduction of phytochemical content of American hemp is certainly an injustice to the medicinal value of this newly-founded commercial crop, the pains associated with the new 0.3% total THC regulation also resonate with commercial growers. As illustrated in Table I, the amount of CBD isolate that can be derived from a given acreage is directly correlated with the CBD content in the plant material being cultivated. And while our own research has revealed that a final CBD concentration of 10% w/w represents the limit of compliance under the 0.3% total THC guideline, many farmers aren’t willing to push this threshold to potentially face regulatory enforcement (controlled crop destruction, fines, or incarceration), and as such produce safe, yet inferior, plants (Table I, left scenario). Unfortunately, this has a tremendous impact on operations, logistics, expenditures, and the environment. Growing “weaker” plants requires that a larger acreage be utilized to produce enough CBD for a set amount of isolate. This equates to large capital expenditures (real estate, equipment, labor), and the use of more water, nutrients, and power. For example, those operating under the jurisdiction of the 1.0% total THC guideline are effectively able to produce as much as five times the amount of hemp-based medicine in a given space compared to those following the 0.3% total THC requirement (Table I). Outdoor cultivation is often regarded as a more environmental-friendly form of hemp cultivation compared to indoor or greenhouse cultivation based on the simple fact that supplemental lighting and HVAC are not required to grow hemp in the open atmosphere. Nonetheless, the need to occupy more land, use more water, and spread more exogenous nutrients to facilitate the cultivation of 5–10% CBD hemp, almost negates the environmental stewardship of outdoor cultivation.
Additionally, larger commercial cultivation facilities present a larger degree of risk. Inherently, larger farms are more susceptible to rogue pollination events than small farms; the occurrence of which can severely compromise yield and phytochemical production as pollinated plants will divert cellular resources to the production of seeds as opposed to the expression of cannabinoids and terpenes. The management of larger allotments of vegetative matter also has a greater potential for spoilage and loss in the harvesting, drying, and curing phases. This very phenomenon has been an Achilles’ heel in the American hemp industry whereby several entities have experienced significant crop losses due to inexperience with large scale drying.
Lastly, the production of less phytochemically-dense hemp material also makes distribution and logistics a challenge for farmers. If the plant material is produced with the intention to generate oils, concentrates, or isolates, then many extractors will demand a much more substantial split of any profit sharing since the margins to produce a viable end product are so thin. Conversely, if the hemp flower is cultivated for sale as “smokeable” flower, every percentage point of CBD or total terpenes that is lost renders the commodity less valuable.
Conclusions
Collectively, the implementation of the 0.3% total THC regulation in the American hemp industry has made the production of CBD-rich hemp more expensive, more precarious, and less lucrative for farmers. Generally, the goal of having regulatory framework in place is to help facilitate the production of consistent products that are reliable and safe. However, when the regulatory entities promulgate new regulations that are unsubstantiated and are not based on the latest scientific data, we find ourselves in a perilous situation that hinders quality, dissuades innovation, and makes hemp cultivation seem like a fool’s errand. Our hope with this commentary is to convey the importance of taking genetics and available horticultural data into account when trying to regulate this new frontier of medicine. Implementing more accommodating cultivation requirements, such as the 1.0% total THC requirement utilized in parts of Europe, will not adversely affect product safety or increase the risk of producing hemp products that trigger significant psychoactivity. On the contrary, these more favorable guidelines present farmers with a greater opportunity for success, and would make hemp cultivation a more sustainable pursuit from both a financial and environmental perspective.
About the Authors
ADAM JACQUES is with ZED Therapeutics. ZACARIAH HILDENBRAND is with Curtis Mathes Corporation.
Please send correspondence to
adam@zedtherapeutics.com
How to Cite this Article
A. Jacques and Z. Hildenbrand, Cannabis Science and Technology 4(5), 22-24 (2021).
