Hemp Testing Insanity II: Impending Hemp Testing Harvest Season Challenges
How can we solve hemp testing problems such as representative sampling, composite samples, and insuring there will be enough testing capacity to meet the demand?
The insanity of different states using different sampling schemes, analytical methods, different analytes, and different concentrations to regulate hemp growers has been discussed here before. With the promulgation of the U.S. Department of Agriculture’s Interim Final Rule in October 2019, some clarity has been brought to the problem. However, there are still problems with representative sampling, composite samples, and insuring there will be enough testing capacity to meet the demand for testing come harvest season. Here, some thoughts on how to solve this problem are shared.
In a recent column (1), I outlined the problem of different states using different sampling schemes, analytical methods, different analytes, and different concentrations to regulate hemp growers. With the promulgation of the United States Department of Agriculture’s (USDA) Interim Final Rule in October 2019 (2), some clarity has been brought to the problem. However, there are still problems with representative sampling, method differences, and insuring there will be enough testing capacity to meet the demand for testing come this harvest season. Some thoughts on how to solve this problem are discussed below.
Why Is Getting Hemp Testing Right so Important?
The answer: Because cannabidiol (CBD) is medicine and it should be tested like medicine. Hemp is an unusual crop because if it is being grown for CBD, rather than for its traditional uses as food and fiber, farmers are growing medicine. This is in part why the industry is so highly regulated. The absolute limit of 0.3% total tetrahydrocannabinol (THC) means that a farmer’s ability to sell their crop and make a living is affected by changes in total THC content of a few hundredths of a percent. I know of no other crop where such high stakes testing is required by law. Some farmers have literally bet the farm on hemp, weren’t able to sell their crops, and committed suicide as a result (3). Since Federal legalization of hemp (4) thousands of farmers across the US have taken the risk and invested heavily into planting hemp crops. We owe it to them, the backbone of our industry, to give them the best testing data and the fairest chance of being able to sell their crop.
Effects of USDA Interim Final Rule for Hemp Testing
My first article on hemp testing insanity was published in the October 2019 edition of Cannabis Science and Technology (CST) (1). In it I lamented the lack of guidance on hemp testing and how the variation in methodologies across states and third party laboratories produces inconsistencies in test results that are not fair to hemp farmers. As if they heard my call, within days of my article going to press the USDA promulgated an Interim Final Rule (IFR) for the regulation of the hemp industry nationwide (2). As far as hemp testing goes, the interim final rule made the five points below clear.
1. The Cannabinoid Being Regulated Is Total THC
Total THC is determined using this equation
Total THC = 0.877(THCA) + Î-9 THC [1]
and the amount of total THC is not allowed to exceed 0.3% by dry weight. This is useful since, as I have pointed out (1), some states were regulating on total THC, others on THC, and others some combination of the two.
2. Margin of Error Has Been Taken into Account
The IFR defines an “acceptable hemp THC level” by adding the margin of error to 0.3%. For example, if for a given analyzer the error for the determination of total THC in dried hemp is ±0.04 weight percent, the acceptable hemp THC level for samples measured on that analyzer would be 0.34 weight percent total THC or less. This is welcome since all measurements contain error (5) so this must be taken into account when determining hemp legality. Previously, some states were not taking margin of error into account and were insisting on a strict 0.3% total THC limit, whereas others were allowing upwards of 0.1% error and passing samples that were upwards of 0.4% total THC.
3. A Representative Sample Must Be Collected
The suggestion is to take one cutting per acre, and that cutting should be from the top third of the plant. I will have more to say about this further on.
4. Proposal to Implement a Nationwide Hemp Testing Laboratory Licensing Program
Please, Dear God, yes do this now please! I have been speaking, researching, and writing upon the inter-laboratory variation problem in this industry for some time (5–9). Briefly, the problem is different laboratories report significantly different results on the same samples. This is caused by human error, a lack of standard analytical methods, and the absence of appropriate standard reference materials. Inter-laboratory variation is a significant problem for our industry, and the only way to solve it is governmental oversight imposing scientifically correct practices and standard methods and samples on the industry. A federal laboratory licensing program is a step in the right direction.
5. Gas Chromatography, Liquid Chromatography, and Alternative Methods May Be Used for Testing
I bring this up because I have had people tell me that the USDA requires gas or liquid chromatography be used for hemp testing. This is not true as I have confirmed with in-person meetings with the science staff at the USDA. The whole point of the phrase “alternative methods” is that the USDA realizes that nonchromatographic technologies exist or may be developed to test hemp that are just as viable as gas chromatography (GC) or high performance liquid chromatography (HPLC). For example, there exist infrared spectroscopic methods for analyzing hemp that are in use (9,10). To be clear, the USDA does not test hemp, does not have a hemp testing method, and does not approve or disapprove of any given hemp testing method. If people mistakenly force themselves to only use chromatography for hemp testing, they are denying themselves the benefits of alternative technologies.
2020 Growing Season Hemp Testing Challenges
However, even with the issuance of the IFR, I still see challenges that are going to affect the testing of our hemp crop in 2020. As we went to press, different states are submitting hemp testing proposals to the USDA for approval. The proposals I have seen from different states vary significantly. This by itself is cause for concern. How can we compare results across states if different methods are used? Here are the challenges as I see them.
Challenge #1: Representative Sampling
Hemp is a naturally variable, inhomogeneous material. Cuttings from adjacent plants, and cuttings from the same plant, can vary in cannabinoid content by several percent. This means we cannot take just one cutting from a field, analyze it, and expect it to yield representative results for the entire plot. The probability of this one cutting accurately representing a field as a whole is quite low. I have written in this journal on the importance of representative sampling in the cannabis industry (10), and so has my fellow CST columnist Patricia Atkins (11).
The problem we are faced with here, trying to characterize a diverse population, is similar to the task that pollsters face trying to determine the opinion of the American populace. The solution pollsters use is to choose people at random, gather data from them, average the data, and then report the results with a margin of error. In this case, the margin of error is a sampling error, and the more people included in a poll the smaller the sampling error becomes.
Similarly, for a hemp grow samples should be selected at random, each of them chemically analyzed, the data averaged, and the results reported with an appropriate margin of error.
A concern I have is sampling plans that collect based on acreage rather than the number of plants. Choosing the number of samples based on acreage is like a pollster interviewing the same number of people in a small town versus a big city because their jurisdictions cover similar physical areas. This would lead to interviewing the same number of people in New York City (NYC), population 8 million, versus somewhere like the village of Bergen, NY (my hometown) with a population 1240 (12). This is not correct because the handful of opinions gathered from NYC will not be representative of the city as a whole. Additionally, when the data are averaged the opinions of Bergenites will have an impact on the results wholly out of proportion to their numbers.
Another problem with determining the number of samples based on hemp acreage is that different grows have different numbers of plants per acre. Farmers may plant 1000, 2000, or more plants per acre. The population we are monitoring here is the plants, not the surface area taken up by them. The scientifically and statistically correct thing to do here then is to adjust the number of samples collected to the number of plants in the plot being monitored. I want our industry to set for itself the goal of analyzing one plant per every 1000 planted. This may sound laughable, but new developments in chemical analysis make this much more feasible than you may think (13,14) as I will discuss below.
Challenge #2: Composite Samples Are Not Representative or Uniform
Another challenge I see with the proposed testing regimens is the collection and analysis of composite samples. There are plans out there that call for collecting many samples from a field in a scientifically correct way. . . yes, let’s keep doing that! However, all these samples are then thrown together to make one composite sample, and then only one aliquot of that composite sample is analyzed. This is like asking one person who they support for President and then saying that one opinion represents the entire country. Of course this is not representative, and neither is analyzing one aliquot of any sample. The correct approach here would be to analyze each of the individual samples collected.
Another problem with the composite sample approach is that it is assumed that the composite sample is uniform. I have seen no data to support this. My own work on this subject is seen in Table I. Five ground, homogenized composite hemp samples representing different strains were analyzed in triplicate via HPLC at a state licensed, ISO certified third party testing laboratory, SC Labs in Santa Cruz, CA (15). The total CBD values obtained on these samples are listed in the table.
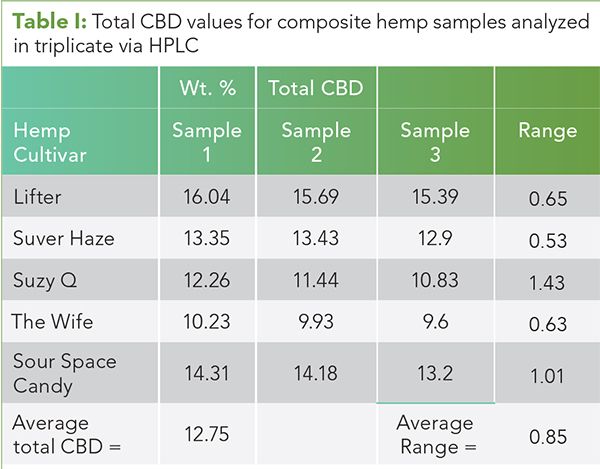
Note that the average range of values for the five strains is 0.85 wt.%, and that the average value of total CBD for the 15 samples is 12.75 wt.%.
If each composite sample were truly uniform there would be no sampling error and the range of values for a given sample should be equivalent to the measured error for the HPLC method. According to the certificates of analysis issued by SC Labs, the error in their method is about ±0.05 wt.%. This is 17 times smaller than the average range in total CBD for the five samples of 0.85 wt.%. We must then conclude that there is significant sampling error here, and that these composite samples are not uniform. The practice then of collecting composite samples and only analyzing one aliquot from them should be avoided.
Challenge #3: Testing Capacity
As stated above, I want to see the hemp industry do the statistically and scientifically correct thing and analyze one hemp plant per 1000. How many tests is that? Sources on the exact amount of hemp acreage planted in 2019, and the plant density used give conflicting data. For the sake of argument let’s use a conservative estimate, 100,000 acres of hemp planted at a density of 2000 plants/acre. That means approximately 200 million hemp plants were grown in the United States in 2019. If we test one plant per 1000 that is 200,000 tests; that’s a lot of tests. Another challenge is that all of these tests have to be performed during a short harvest season that lasts a few months, not an entire year. Given that the onus for performing hemp compliance testing rests on the states, from my knowledge there is not currently enough testing capacity in many states to handle this testing burden? What should we do?
A Potential Solution
In a previous column, I introduced the chemical analysis techniques of chromatography and spectroscopy, and compared and contrasted them for cannabis potency analysis (16). In that column, I discussed the four figures of merit for chemical analysis methods: speed, accuracy, cost, and representative sampling. Chromatography had the advantage of accuracy, but struggled with speed and cost. For example, it can take up to 20 min to weigh, grind, measure out and add solvent, mix, filter, dilute, shoot, and analyze a single sample by chromatography. When the costs of vials, filters, solvents, solvent disposal costs, and labor are taken into account it can cost $30 or more per analysis (16). Also, common sense and state regulations require that chromatographs be operated only by highly trained technicians and scientists. I believe these limitations are why we are not sampling hemp in a representative manner; it is not lack of desire but the obstacle of chromatography making the level of testing required too slow and expensive. In theory a massive investment in chromatographs and training programs could get us to our goal of sampling one plant per every 1000, but at a significant cost.
Spectrometers, since they are calibrated using chromatographic reference data, will be somewhat less accurate than chromatographs (16). However, there exist spectroscopic analyzers that can analyze hemp accurately enough to determine whether a simple is above or below the 0.3 wt.% total THC limit with little sample preparation, a 2 min analysis time, at a cost of $0/sample (12,13). These instruments are capable of analyzing hundreds of samples per day, and are easy enough to use that most anyone can be trained to use them. Spectrometers then can help increase our testing capacity, promote more representative sampling, and give hemp farmers the best data we can so they have the fairest chance of being able to market their crop. Wider use of these instruments should be considered by regulators and farmers.
Conclusions
It is important to test our hemp crop as best as we can since CBD is medicine, and people’s livelihoods depend upon accurate testing. Recent USDA rulemaking has somewhat clarified the situation. However, hemp testing plans being proposed by states for USDA approval suffer from improper sample collection, the assumption of composite samples being uniform, and an overdependence on slow and expensive chromatographic analyses. These problems will prevent us from analyzing one plant per 1000 as is statistically and scientifically correct. Recent developments in spectroscopic systems enable hemp to be analyzed quickly, at low cost, and with sufficient accuracy to be useful. Hemp regulators and farmers need to consider these systems when developing hemp testing programs.
References:
- B.C. Smith, Cannabis Science and Technology2(5), 10–13 (2019).
- https://www.federalregister.gov/documents/2019/10/31/2019-23749/establishment-of-a-domestic-hemp-production-program.
- https://mailtribune.com/news/top-stories/not-all-southern-oregon-hemp-farmers-struck-gold.
- 115th United States Congress, Senate Bill S.2667,” Hemp Farming Act of 2018.
- B.C. Smith, Cannabis Science and Technology1(4), 12–16 (2018).
- B.C. Smith, P. Lessard, R. Pearson, Cannabis Science and Technology2(1), 48–53 (2019).
- B.C. Smith, Cannabis Science and Technology2(2), 12–17 (2019).
- B.C. Smith, Cannabis Science and Technology2(3), 10–14 (2019)
- B.C. Smith, Cannabis Science and Technology3(2), 10–15 (2020).
- B.C. Smith, Cannabis Science and Technology2(1), 14-19 (2019).
- P. Atkins, Cannabis Science and Technology2(2), 26–34 (2019).
- https://en.wikipedia.org/wiki/Bergen_(village),_New_York.
- B.C. Smith, Cannabis Science and Technology2(6), 28–33 (2019).
- C. Sánchez-Carnerero Callado, N. Núñez-Sánchez, S. Casano, and C. Ferreiro-Veraa, Talanta190, 147–157 (2018).
- https://www.sclabs.com/.
- B.C. Smith, Cannabis Science and Technology2(6), 10–14 (2019).
About the Columnist

Brian C. Smith, PhD, is Founder, CEO, and Chief Technical Officer of Big Sur Scientific in Capitola, California. Dr. Smith has more than 40 years of experience as an industrial analytical chemist having worked for such companies as Xeros, IBM, Waters Associates, and Princeton Instruments. For 20 years he ran Spectros Associates, an analytical chemistry training and consulting firm where he improved their chemical analyses. Dr. Smith has written three books on infrared spectroscopy, and earned a PhD in physical chemistry from Dartmouth College.
How to Cite this Article
B.C. Smith, Cannabis Science and Technology3(3), 12–15 (2020).
