Measuring Heavy Metals in Cannabis: What Atomic Spectroscopic Technique Is Right for Your Laboratory?
This article takes a look at the major atomic spectroscopy techniques and compares their performance characteristics and in particular examines the limits of quantitation for the four heavy metals (Pb, As, Cd, and Hg) currently being regulated in cannabis and hemp by most states in the US.
Since the introduction of the first commercially available flame atomic absorption spectrophotometer (FAAS) in the early 1960s, there has been an increasing demand for better, faster, easier-to-use, and more flexible trace element instrumentation. A conservative estimate shows that in 2019, the market for atomic spectroscopy-based instruments was well in excess of $1 billion in annual revenue, and that doesn’t include after-market sales and service costs, which could increase that number by an additional $500 million. As a result of this growth, we have seen a rapid emergence of more sophisticated, easier-to-use equipment. Moreover, with an increase in the number of manufacturers of atomic spectroscopy instrumentation and its sampling accessories, together with the availability of traditional solid sampling techniques—such as X-ray fluorescence (XRF) and newer techniques such as microwave-induced optical emission spectroscopy (MIP-OES), laser induced breakdown spectroscopy (LIBS), and laser ablation laser ionization time-of-flight mass spectrometry (LALI-TOFMS)—the choice of which technique to use is often unclear. It also becomes even more complicated when budgetary restrictions allow only one analytical technique to be purchased to solve a particular application problem. This article takes a look at the major atomic spectroscopy techniques and compares their performance characteristics and, in particular, examines the limits of quantitation for the four heavy metals (lead [Pb], aresnic [As], cadmium [Cd], and mercury [Hg]) currently being regulated in cannabis and hemp by most states in the US. Note: A more expanded version of this article can be found in my book, Measuring Heavy Metal Contaminants in Cannabis and Hemp (1).
Optimum Technique
To select the best technique for a particular analytical problem, it is important to understand exactly what the problem is and how it is going to be solved. For example, if the requirement is to monitor copper at percentage levels in a copper plating bath and it is only going to be done once per shift, flame atomic absorption (FAA) would adequately fill this role. Alternatively, when selecting an instrument to measure 24 elemental impurities in pharmaceutical products according to United States Pharmacopeia (USP) Chapters <232> (2), <233> (3), and International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Q3D Guidelines (4), this application is clearly better suited for a multielement technique such as inductively coupled plasma-optical emission spectrometry (ICP-OES) or inductively coupled plasma-mass spectrometry (ICP-MS). However, if the demand is for four heavy metals in cannabis or hemp the decision is not so clear. ICP-MS could be the most suitable technique, but depending on the state maximum limits, the cannabis product being tested, the sample workload, or budgetary restrictions, other techniques such as graphite furnace atomic absorption (GFAA) or vapor generation atomic fluorescence (VGAF) might be the best fit.
So when choosing a technique, it is important to understand not only the application problem, but also the strengths and weaknesses of the technology being applied to solve the problem. However, there are many overlapping areas between the major atomic spectroscopy techniques, so it is highly likely that for some applications, more than one technique would be suitable. For that reason, it is important to go through a carefully thought-out evaluation process when selecting a piece of equipment (5).
The main intent of this article is therefore to look at measuring the big four heavy metals in cannabis and cannabis products to offer some insight as to which might be the most suitable atomic spectroscopic approach. We will not be discussing XRF, MIP-OES, LIBS, or LALI-TOF-MS techniques in this evaluation. They are all very useful techniques for many sample types and have been described at length in the open literature (6, 7). Chapter <233> does allow the use of an alternative technique, as long as it meets the validation and verification protocols described in the analytical procedure section. In fact it has been shown that XRF is a valid technique to use as a screening tool to reduce the number of samples that need to be analyzed by one of the spectrochemical techniques (8). However for the purpose of this comparison, we will be focusing on the most commonly used atomic spectroscopy techniques—flame atomic absorption (FAA), GFAAA, hydride generation atomic absorption (HGAA), VGAF, ICP-OES, and ICP-MS.
Basic Principles of Atomic Spectroscopy
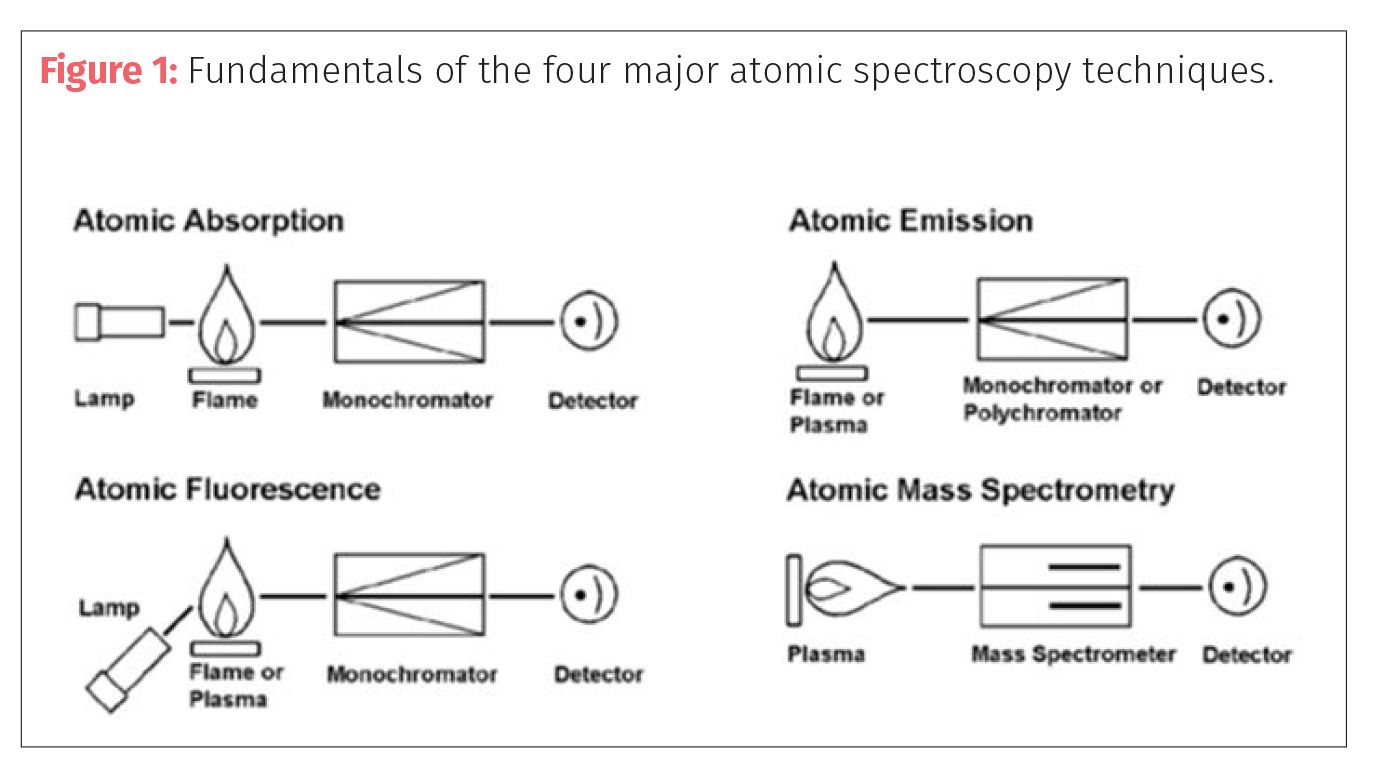
The fundamental principles of these four techniques are represented in Figure 1.
FAA is predominantly a single-element technique that uses a flame to generate ground-state atoms. When light in the form of a hollow cathode or electrodeless discharge lamp is passed through this atom cloud, specific wavelengths of light are absorbed that are characteristic of the metal of interest. The amount of light absorbed is indicative of the amount of analyte present. Flame AAS is capable of low parts per million (ppm) detection limits.
HGAA is a very useful analytical technique to determine the more volatile, hydride forming metals, such as As, Bi, Sb, Se, and Te. In this technique, the analytes in the sample matrix are first reacted with a very strong reducing agent, such as sodium borohydride to release their volatile hydrides, which are then swept into a heated quartz tube for atomization. The tube is heated either by a flame or a small oven that creates the ground state atoms of the element of interest, which are then measured by atomic absorption. HGAA is capable of approximately three orders of magnitude lower detection limits than FAA for the hydride forming elements.
Cold vapor atomic absorption (CVAA): The determination of elemental mercury can also be carried out using reduction chemistry. This is known as the cold vapor (CV) technique and by generating elemental mercury in the form of a vapor; it can improve the detection for mercury over FAA by up to 4 orders of magnitude. It should also be pointed out that dedicated mercury analyzers using atomic fluorescence are capable of even lower detection limits.
Atomic fluorescence (AF) coupled with hydride generation (HG) or cold vapor generation (VG) can also be used to improve the detection capability of the volatile elements. Atomic fluorescence shares properties with atomic absorption in that it has a light source, atomizer, monochromator, and detector. The element specific light energy is absorbed, taking the element through excitation. However, rather than determining the amount of light being absorbed by the element in the atomizer, as the excited atoms return to ground state, they emit photons of light and the emission (or fluorescence) resulting from the decay is measured. The major difference is the element specific light source is at an angle so as not to interfere with the fluorescence process. To carry out multielement analysis using AFS, many lamps are positioned around the atomization or excitation source. Atomic fluorescence coupled with hydride or vapor generation can improve the detection limits compared to FAA by up to 3–4 orders of magnitude, but with the added benefit of being multi-element, based on the number of lamps around the excitation source. There are in fact commercially-available vapor generation AF systems on the market, which are being used for the measurement of Pb, Cd, As, and Hg in cannabis samples (9, 10).
GFAA is also a single element technique, although some multielement instrumentation is now available. GFAA works on exactly the same principle as FAAS, except that the flame is replaced by a small heated graphite tube to generate the source of atoms. Because the ground state atoms are concentrated in a smaller volume than a flame, more light absorption takes place resulting in detection limits approximately 100–1000 times lower than for FAAS.
ICP-OES is a multielement technique that uses an extremely hot plasma source to excite atoms to the point where they emit wavelength-specific photons of light, characteristic of each particular element. The number of photons produced is directly related to the concentration of that element in the sample. ICP-OES instrumentation comes in two configurations, radial and axial. The radial configuration uses a traditional vertical plasma source where the emitting light is viewed from the side, whereas the axial configuration uses a horizontal plasma, where the light is viewed directly down the center. The benefit of the axial design is that more photons are seen by the detector and as a result offers 5–10 lower detection limits than the radial configuration. ICP-OES is capable of low parts-per-million to parts-per-billion detection limits.
ICP-MS also uses a plasma source. However, the fundamental difference between ICP-OES and ICP-MS is that the plasma is not used to generate photons of light, but to generate positively-charged ions. The ions produced in the plasma are transported and separated by their atomic mass to charge ratio, using a mass spectrometer. The generation of such large numbers of positively charged ions allows ICP-MS to achieve detection limits for most elements at the parts-per-trillion level.
Comparison Highlights
There is no question that the multielement techniques like ICP-OES and ICP-MS are better suited for the determination of the elements defined in USP Chapter <232>, especially in laboratories that are expected to be carrying out the analysis in a high throughput, routine environment. If the best detection limits are required, ICP-MS offers the best choice followed by GFAA. Axial ICP-OES offers very good detection limits for most elements, but generally not as good as GFAA. Radial ICP-OES and FAA show approximately the same detection limits performance, except for the refractory, rare earth, and transuranic elements, for which performance is much better by ICP-OES. For Hg and those elements that form volatile hydrides, such as As, Bi, Sb, Se, and Te, the HGAA or VGAF techniques offer exceptional detection limits. Figures 1 and 3 and Table I show an overview of the detection capability, working analytical range and sample throughput of the major atomic spectroscopy approaches, which are three of the metrics most commonly used to select a suitable technique.
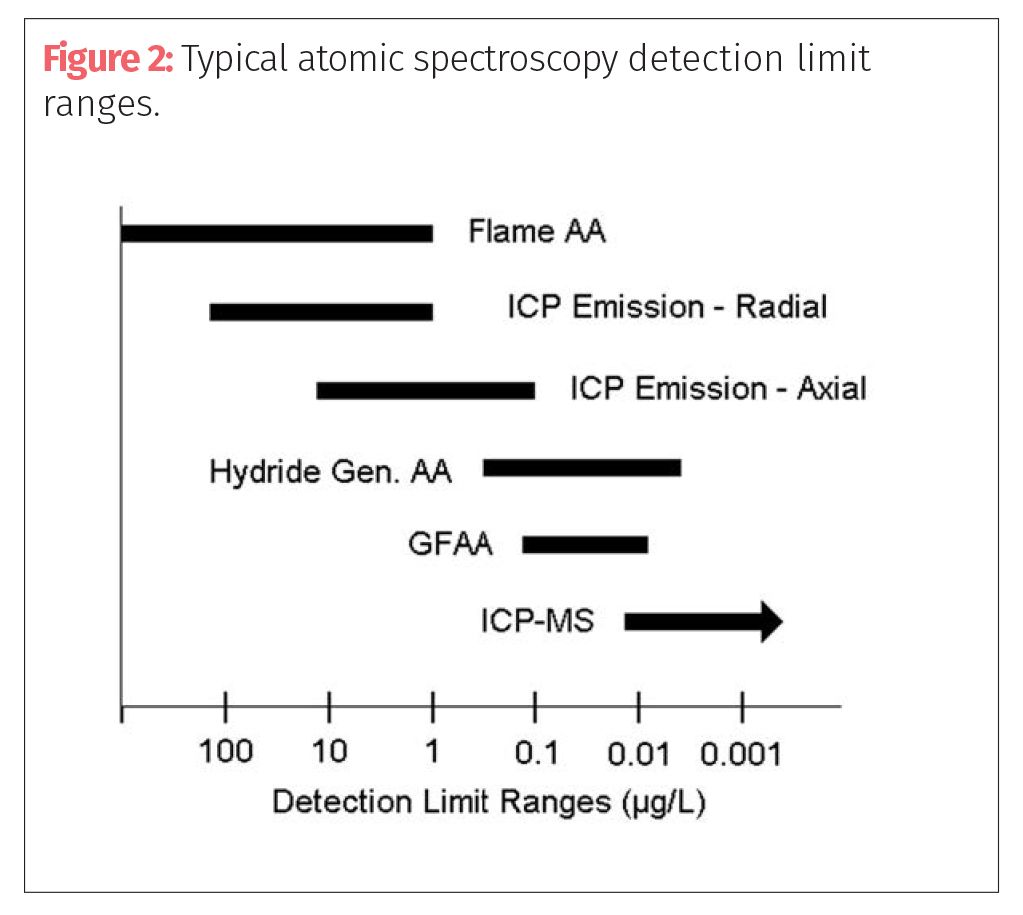
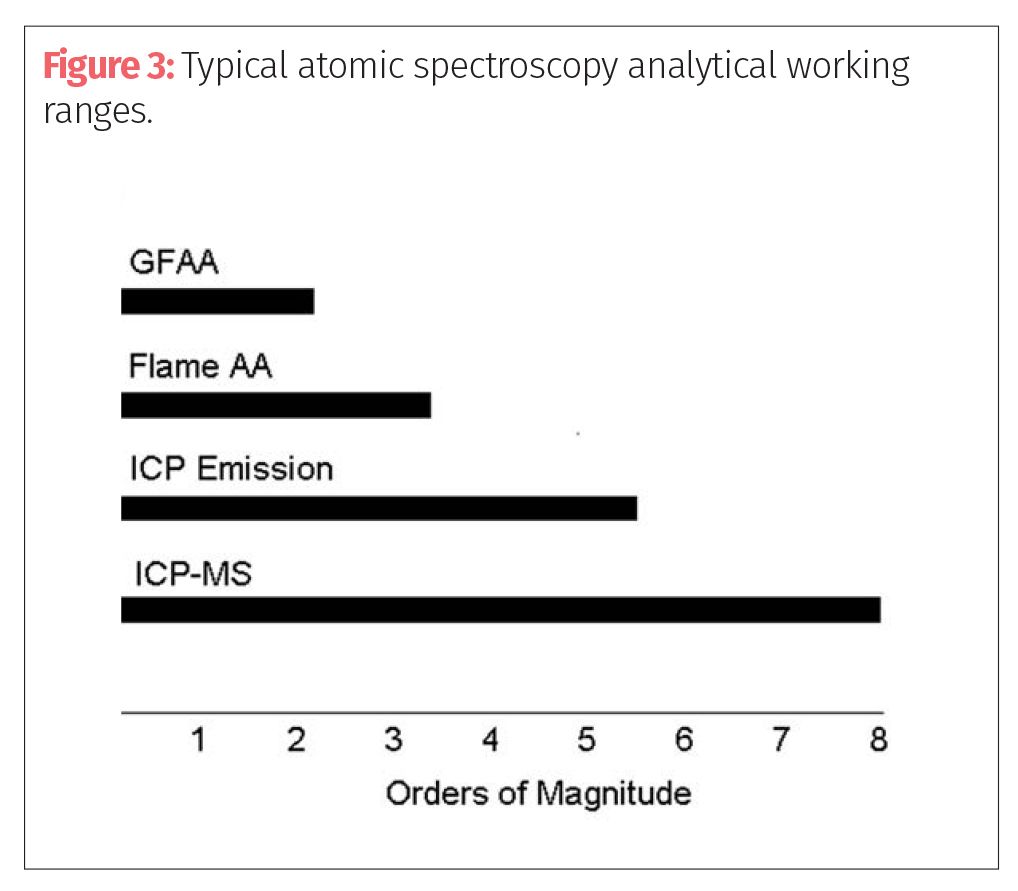
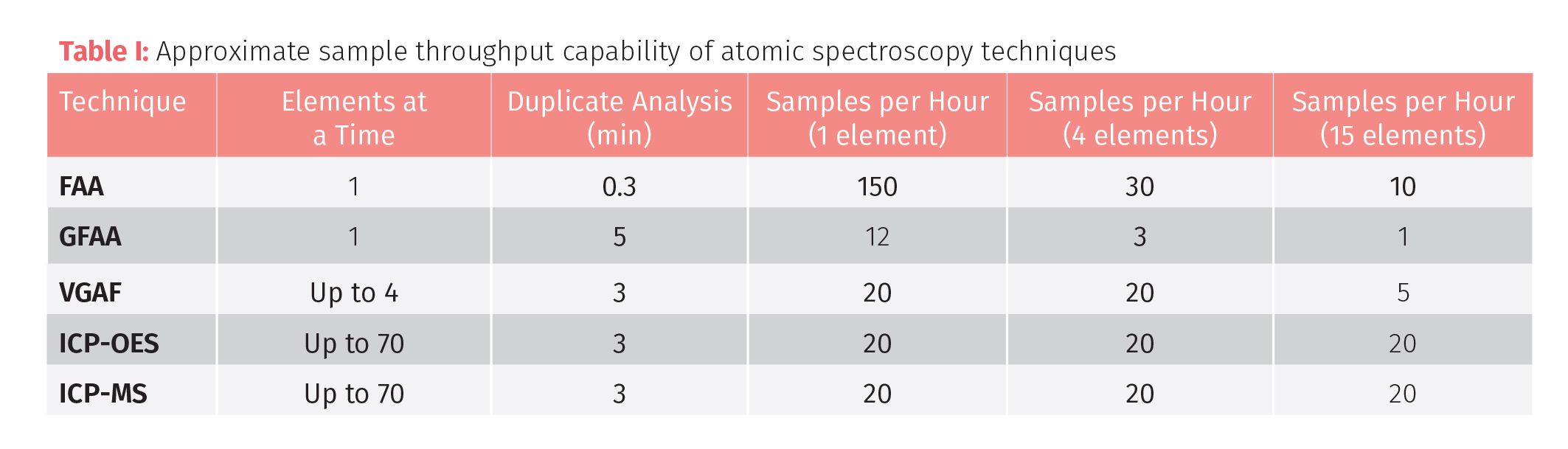
This is not meant to be a detailed description of each technique, but a basic understanding as to how they differ from each other. Let’s now turn our attention to selecting the best technique based on the demands of the cannabis and hemp testing community.
Demands of the Cannabis Industry
Comparing an instrumental technique based on its performance specifications with simple standards is important, but it bears little relevance to how that instrument is going to be used in a real-world situation. For example, instrument detection limits (IDL) are important to know, but how are they impacted by the matrix and sample preparation procedure? So, what are the real-world method detection limits (MDL) and is the technique capable of quantifying the maximum concentration values expected for this analysis? And what kind of precision and accuracy can be expected if working close to the limit of quantitation (LOQ) for the overall methodology being used? Additionally, on the sample throughput side, how many samples are expected and at what frequency will they be coming into the laboratory? How much time can be spent on sample preparation and how quickly must they be analyzed? Sometimes the level of interferences from the matrix will have a major impact on the selection process or even the amount or volume of sample available for analysis.
Understanding the demands of an application is therefore of critical importance when a technique is being purchased, and particularly if there is a minimum amount of expertise or experience in house on how best to use it to solve a particular application problem. This could be the likely scenario in a cannabis testing laboratory that is being asked to check the four elemental contaminants in cannabis, or one of its many cannabinoid products. Because there are currently very few regulated methods available, the industry is turning to the highly regulated pharmaceutical industry for guidance. As a result, they use the new USP Chapter 232 or ICH Q3D Guidelines to carry out the measurements, which recommend the use of a plasma-based spectroscopic technique or any other atomic spectroscopy technique as long as it meets the validation protocols described in USP Chapter 233.
The expertise of the operator should never be underestimated because, if ICP-MS is being seriously considered, it generally requires an analyst with a higher skill level to develop rugged methodology free of interferences that can eventually be put in the hands of an inexperienced user to operate on a routine basis. It also requires someone with the knowledge of working in the ultra-trace environment where understanding contamination issues and laboratory cleanliness is of the utmost importance. This again is a real concern if the technique is being used by novice users who have limited expertise in running analytical instrumentation, which may be the case in the cannabis industry. So let’s take a closer look at the permitted daily exposure (PDE) limits defined in USP Chapter <232> and ICH Guidelines to understand what atomic spectroscopy techniques might best meet the demands of measuring the four heavy metals in cannabis. Note: PDE is defined as the maximum acceptable intake of elemental impurity in a pharmaceutical product per day.
Suitability of Technique
USP Chapter <233> is being adopted by most cannabis testing laboratories as the standardized methodology to test cannabinoid-based products for heavy metals. To get a better understanding of the suitability of the technique being used and whether its detection capability is appropriate for the samples being tested, it’s important to know the PDE limits for each of the four target elements and, in particular, what the USP calls the J-value, as described in Chapter <233>. The J-value is defined as the PDE concentration of the element of interest (based on the suggested maximum dosage per day), appropriately diluted to the working range of the instrument, after the sample preparation procedure is completed. Let’s take Pb as an example. The PDE limit for Pb in an oral medication (or an orally-delivered cannabinoid product) defined in Chapter <232> is 5 µg/day.
Based on a suggested dosage of 10 g of the product/day, that’s equivalent to 0.5 µg/g Pb. If 0.2 g of sample is digested or dissolved and made up to 100 mL (typical for many cannabis samples), this equals a 500-fold dilution, which is equivalent to 1.0 µg/L. So the J-value for Pb in this example is equal to 1.0 µg/L.
The method then suggests using a calibration made up of two standards: Standard 1 = 1.5J, Standard 2 =
0.5 J. So for Pb, that’s equivalent to 1.5 µg/L for Standard 1 and 0.5 µg/L for Standard 2.
The suitability of a technique is then determined by measuring the calibration drift and comparing results for Standard 1 before and after the analysis of all the sample solutions under test. This calibration drift should be <20% for each target element. However, once the suitability of the technique has been determined, further validation protocols described in Chapter <233> including accuracy, detectability, specificity, repeatability, and ruggedness must be carried out to show compliance to the regulatory agency if required.
It should also be pointed out that no specific instrumental parameters are suggested in Chapter <233>, but only to analyze according to the manufacturer’s suggested conditions and to calculate and report results based on the original sample size. However, it does say that appropriate measures must be taken to correct for interferences, such as matrix-induced wavelength overlaps in ICP-OES and argon-based polyatomic interference with ICP-MS.
Let’s exemplify this by taking an analytical scenario of measuring four elemental contaminants (Pb, Cd, As, and Hg) in a cannabis product that is being regulated according to the USP Chapter <232> and ICH Q3D inhalation PDEs (many states use the inhalation maximum limits even though a cannabis product might be consumed orally because the inhalation values are much lower) and calculate the J-values for each heavy metal and compare them with the limits of quantitation (LOQ) for each technique to give us an assessment of their suitability. For this analytical scenario, we’ll take the LOQ for the technique as 10x the IDL. These LOQs were calculated by taking the average of published IDLs from three instrument vendors’ application material and multiplying them by 10 to get an approximation of LOQ. In practice, a method limit of quantitation is typically determined by processing the matrix blank through the entire sample preparation procedure and taking 10 replicate measurements. The method LOQ, sometimes referred to as the method detection limit (MDL), is then calculated as 3–7x standard deviations of these 10 measurements, depending on the % confidence level required.
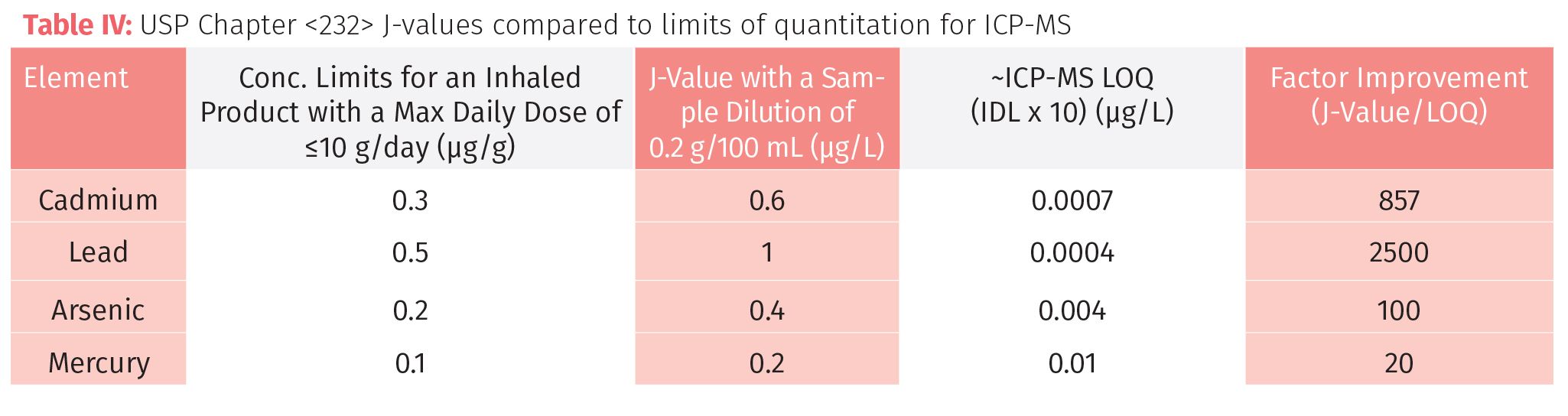
To make this comparison valid, the sample weight was adjusted for each technique, based on the detection limit and analytical working range. So for AA, VGAF, and ICP-OES we based the calculation on a dilution of 2 g/100 mL (or 0.5 g/25 mL), whereas for ICP-MS we used 0.2 g/100 mL. AA and ICP-OES could definitely use larger sample weights, but for high throughput routine analysis, we are probably at the optimum dilution for ICP-MS. Tables II, III, and IV show the comparison of AA (FAA and GFAA), VGAF, ICP-OES, and ICP-MS, respectively, for four elemental contaminants in an inhaled cannabis product according to Chapter <232>. The relevant comparative data to consider is in the final column, labelled “Factor Improvement,” which is the J-value, divided by the LOQ. Generally speaking, the higher this number, the more suitable the technique is.
Relationship Between LOQ and J-Value
It should be emphasized again that LOQ in these examples is just a guideline as to the real-world detection capability of the technique for this method. However, it does offer a very good approximation as to whether the technique is suitable based on the factor improvement number compared to the J-values for each elemental impurity. Clearly, if this improvement number is less than 1, as it is with Pb and Cd by FAA, the technique is just not going to be suitable. Although, As and Hg could be improved using hydride or cold vapor techniques. On the other hand, GFAA would be suitable for all the heavy metals. However, the GFAA technique is very time-consuming and labor intensive, so it probably wouldn’t be a practical solution in a high throughput cannabis testing laboratory. On the other hand, VGAF looks to be very promising as the factor improvement for all four heavy metals is between 40 and 600. The comparison between FAA, GFAA, and VGAF is exemplified in Table II.
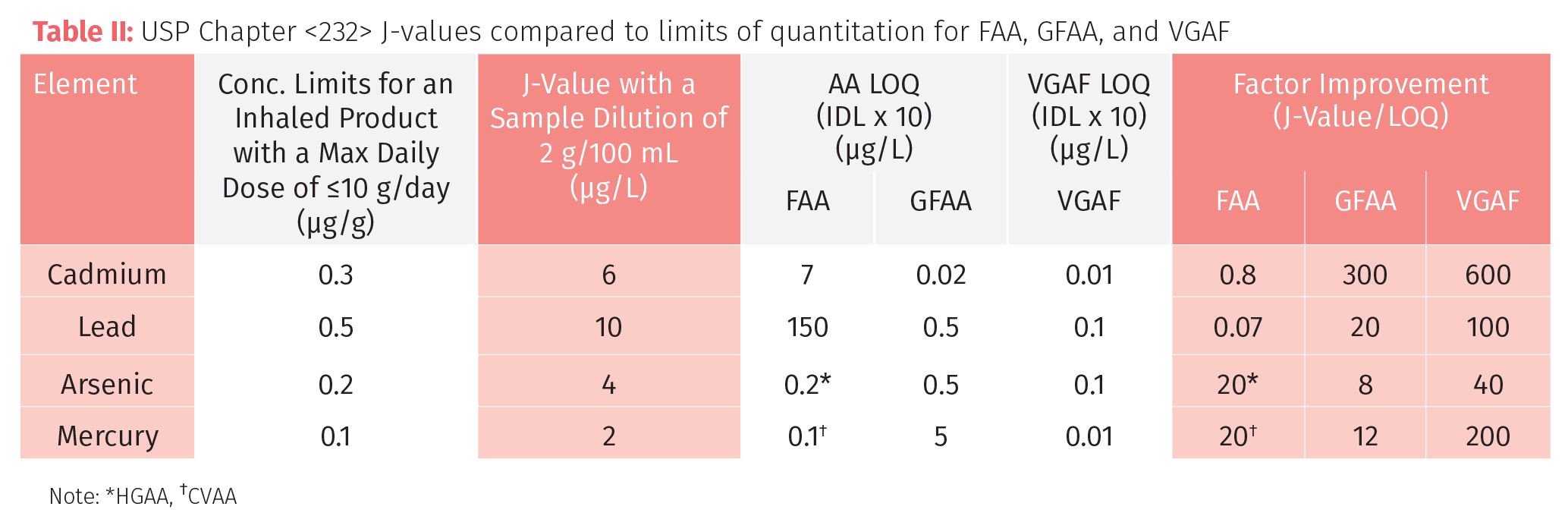
Table III shows that axial-ICP-OES doesn’t look to be a good candidate because aside from Cd, the factor improvements are all around 1 or less. These numbers could be further improved by using a higher sample weight in the sample preparation procedure without compromising the method, or possibly using a sampling accessory such as an ultrasonic nebulizer, which could improve the detection capability by a factor of 5–10 fold. (Note: As most commercial ICP-OES instrumentation offers both axial and radial capability, it was felt that the axial performance was most appropriate for this comparison.)
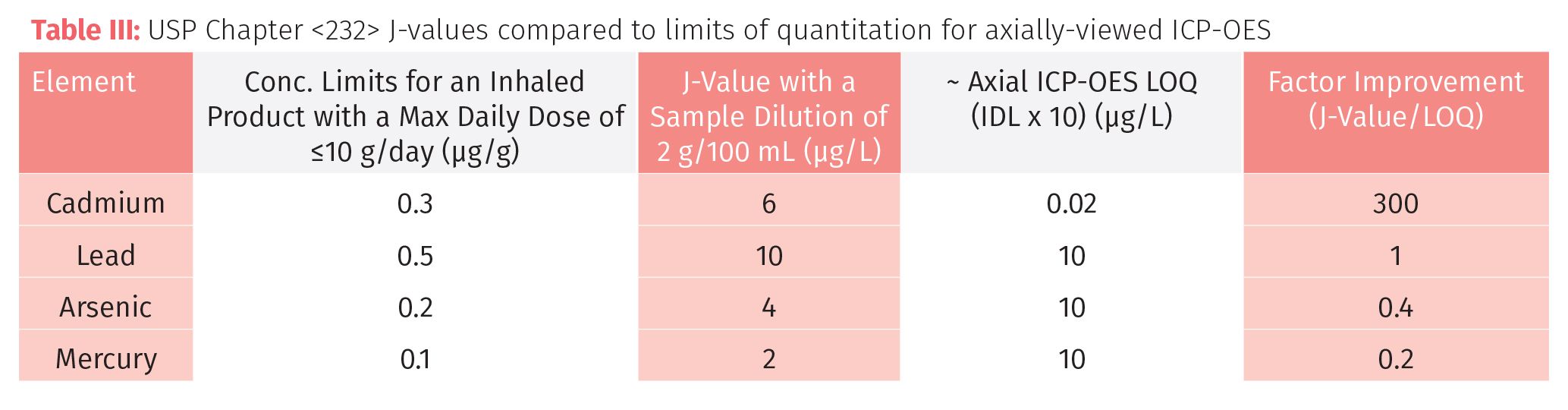
However, it can be seen in Table IV that ICP-MS shows significant improvement factors for all contaminants that are not offered by any other technique. The added benefit of using ICP-MS is that if and when the elemental suite expands to include other heavy metal contaminants, the additional workload should not pose too much of a problem. Additionally, if arsenic or mercury levels were found to be higher than the PDE levels, it would be relatively straight-forward to couple ICP-MS with high performance liquid chromatography (HPLC) to monitor the speciated forms of these elements if required.
Final Thoughts
It is important to understand that there are many factors to consider when selecting a trace element technique most suited to the demands of your application. Sometimes one technique stands out as being the clear choice, whereas other times, it is not quite so obvious. And as is true with many applications, more than one technique is often suitable. However, the current state regulations for cannabis products are based on a mix of USP Chapter <232> inhalation and oral PDEs which presents some unique challenges, not only from a perspective of performance capability, but also because testing laboratories have to meet the validation protocols defined in USP Chapter <233> to show suitability of the technique to the analytical procedure being used. From a practical perspective, there is no question that to meet the lowest limits for inhaled products (used by many state regulators) ICP-MS is probably the most appropriate technique, followed closely by VGAA. For oral cannabis products or transdermal applications, where the maximum limits are higher, axial-ICP-OES combined with performance enhancing sampling accessories could offer a more cost-effective approach. And if the sample workload requirements are not so demanding, GFAA could provide a solution, particularly as solid samples can be analyzed. However, ICP-MS has shown it has the detection limits and throughput capability to be the optimum technique of choice for these types of samples, particularly if the suite of required heavy metals expands at a later date because of stricter federal regulations.
References
- R.J. Thomas, Measuring Heavy Metal Contaminants in Cannabis and Hemp (CRC Press, Boca Raton, Florida, 2020) ISBN 9780367417376.
- General Chapter <232> “Elemental Impurities – Limits,” 1st supplement to United States Pharmacopeia 40–National Formulary 35 (USP40–NF35) (United States Pharmacopeial Convention, Rockville, Maryland, 2017). https://www.usp.org/chemical-medicines/elemental-impurities-updates.
- General Chapter <233> "Elemental Impurities – Procedures," Second Supplement to United States Pharmacopeia 38–National Formulary 33 (USP38–NF33) (United States Pharmacopeial Convention, Rockville, Maryland, 2015). https://www.usp.org/chemical-medicines/elemental-impurities-updates.
- International Conference on Harmonization, ICH Guideline Q3D on Elemental Impurities: Step 5, European Medicine Agency Website: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-13.pdf.
- R.J. Thomas, "Choosing the Right Trace Element Technique: Do you know what to look for," Today Chemist at Work, October, 1999.
- R.J. Thomas, Spectroscopy 29(3), 42–51 (2014).
- J. Williams and J. Putman, Spectroscopy 35(5), 9–16 (2020).
- D. Davis and H. Furukawa, Spectroscopy 32(7), 12–17 (2017).
- Lumina 3500 Atomic Fluorescence Spectrometer, Aurora Illuminating Systems Elemental Analysis Instruments, 2020, https://www.aurorabiomed.com/wp content/uploads/2019/07/LUMINA_3500.pdf.
- PF7 Heavy Metals Analyzer, Persee Analytics Inc., http://perseena.com/index/prolist/pid/33/c_id/43.html.
About the Guest Author

Robert Thomas is the principal of Scientific Solutions, a consulting company that serves the training, application, marketing, and writing needs of the trace element user community. He has worked in the field of atomic and mass spectroscopy for more than 45 years, including 24 years for a manufacturer of atomic spectroscopic instrumentation. He has served on the American Chemical Society (ACS) Committee on Analytical Reagents (CAR) for the past 19 years as leader of the plasma spectrochemistry, heavy metals task force, where he has worked very closely with the United States Pharmacopeia (USP) to align ACS heavy metal testing procedures with pharmaceutical guidelines. Rob has written almost 100 technical publications including five textbooks on the fundamental principles and applications of ICP-MS.
About the Columnist

Patricia Atkins is a Senior Applications Scientist with SPEX CertiPrep and a member of both the AOAC and ASTM committees for cannabis.
How to Cite this Article
R. Thomas, Cannabis Science and Technology 3(9), 21-28 (2020).

Easy GC Injection Set-Up and How to Avoid Peak Splitting of Cannabis Pesticide Samples
May 6th 2025This article will briefly introduce GC splitless inlet anatomy and show a simple example for how optimizing injection type and volume, initial GC oven temperature, and extract dilution can improve GC peak shape.
A Consumer Trends Survey of Minor Cannabinoids
March 6th 2025This survey assesses consumer awareness, usage patterns, and safety concerns with results indicating that cannabidiol (CBD), Δ8-tetrahydrocannabinol (Δ8-THC), and cannabinol (CBN) are the most frequently used cannabinoids, with vapes and edibles being the preferred methods of consumption.