Reading the Leaves: Adventures in the Leaf-Level Physiology of Cannabis
Understanding the biochemical and physical process that comprise and control leaf-level physiology gives us deep insight into the plant, which may convert directly to cultivation practices.
My name is Allison Justice, PhD, and I am the founder and CEO of The Hemp Mine, a vertical cannabidiol (CBD) company in South Carolina. I am a plant scientist by trade, and no plant has ever amazed me as does the cannabis plant. Years of suppression due to federal illegality has kept the discovery of this plant in all realms (chemistry, horticulture, and medicine) almost in its infancy. The industry needs research to grow in legitimacy and efficiency. Due to hemp now being federally legal, universities and other businesses—which prior to recent changes in law would not be involved in the cannabis industry—now have become active. In just the past two years alone, I have been amazed to watch the number of universities becoming involved, graduate students able to use cannabis as a host plant, and businesses marketing to the cannabis industry that once would not. Over the years and back in graduate school, I worked with LI-COR Biosciences on a wide range of host plants. In cannabis, I worked with some of the smaller tools that LI-COR has to offer but never the LI-6800. Being able to operate one of these pieces of equipment was every plant science grad student’s dream. The unit is more expensive than many cars, but the information that can be gathered is beyond what most realize is measurable. So, when the LI-COR team asked if I had plants they could come take some data on, my immediate answer was YES! The point of this article is not a sales pitch as this is a tool that mainly universities and serious researchers would use regularly. The point is to begin to unite research and industry. Though some of this article may seem to come with too much detail, require a degree in Plant Physiology, and not have use to the grower, our hope is to really bring to light the importance of science in the cannabis industry and translate how these data do convert directly to grower benefit.
-Allison Justice, PhD
On the invitation of Dr. Allison Justice this past summer, a colleague, Rod Madsen, and I (Jason Hupp) packed up some instrumentation and took a trip to The Hemp Mine, in Fair Play, South Carolina. The trip was short and the goal simple. We were there to gain some experience in the leaf-level physiology of cannabis.
Both Rod and I are with LI-COR Biosciences, in Lincoln, Nebraska. At the time, he was the product manager for our plant physiology instruments, and I was an application scientist. While our roles were a bit different, we are both deeply involved in the design and development of the kinds of tools researchers all over the globe (and in space!) use to understand how plants function.
Between Rod and I, we have more than 40 years of combined experience in physiological methods and instrumentation. Despite that, neither of us (like most other physiologists) have had much opportunity to work with cannabis. The physiological methods and instrumentation we use were first introduced in the tail end of the 20th century. Their development and widespread application in agricultural and horticultural sciences came along when virtually no agricultural research was being done on cannabis in academia.
Why bother with leaf-level physiology? At first glance, this sort of investigation might seem like something only scientists do for the mere benefit of writing papers that only other scientists will read. This is untrue. Photosynthesis supports everything the plant is and does. Understanding the biochemical and physical process that comprise and control it gives us deep insight into the plant, which may convert directly to cultivation practices.
Think about us like sports physiologists helping to train an Olympic athlete. Imagine the plant as that athlete running on a treadmill, hooked up to monitors while testing their physical limits. The sports physiologist may control what the athlete eats, how they breathe, and how they hydrate, all to try to understand what happens when the athlete is pushed to an extreme. Does their body shut down or is it able to handle the situation? If it does shut down, why? We look at the plant the same way. Our treadmills and monitors look a bit different, but they essentially tell us the same things. We manipulate light, CO2, water, nutrients, and so on, and evaluate how the plant responds in real time, on a photosynthetic level—not just based how it looks or some yield parameter at the end of the growing season.
These measurements of leaf-level physiology find application in a wide range of areas related to plant production. Routine monitoring of physiological parameters is used for early detection of various stressors and to guide management practices. Large datasets are used to build and parameterize models, which in turn serve as predictive tools and test beds for experimental simulations. Evaluation of and selection for many of these physiological traits is standard practice in many plant breeding programs.
This brings us to the key point: this data is highly useful and is virtually nonexistent for cannabis. For most any other high-value or high-volume crop, a quick literature search is apt to return hundreds, if not thousands, of papers with well-defined physiological data at the varietal level. For cannabis, a similar search returns a few dozen papers containing actual measurements and few of those come from material specified below the species level.
What Did We Do at the Hemp Mine?
Dr. Justice gave us access to a mixed age planting of field grown clones of “Janet’s G,” a variety grown primarily for cannabigerol (CBG) production. Over the course of three days, we made a variety of measurements using field portable photosynthesis meters and porometers to explore capacity for and limitations to photosynthesis. What I show here is a small subset of the data we collected while at The Hemp Mine, focusing on some common measurements and what might be their more easily digested highlights.
Response to CO2
CO2 response measurements indicated these plants were phosphate deficient (see Figure 1). For C3 plants, like cannabis, the general pattern of how photosynthetic carbon assimilation—the process of CO2 uptake through photosynthesis—responds to CO2 is well defined. At low concentrations it increases rapidly with increasing CO2, reaching some inflection point after which it typically shows only a gradual increase. This latter behavior happens as excess of CO2 begins to inhibit a process, called photorespiration, which interferes with photosynthesis.
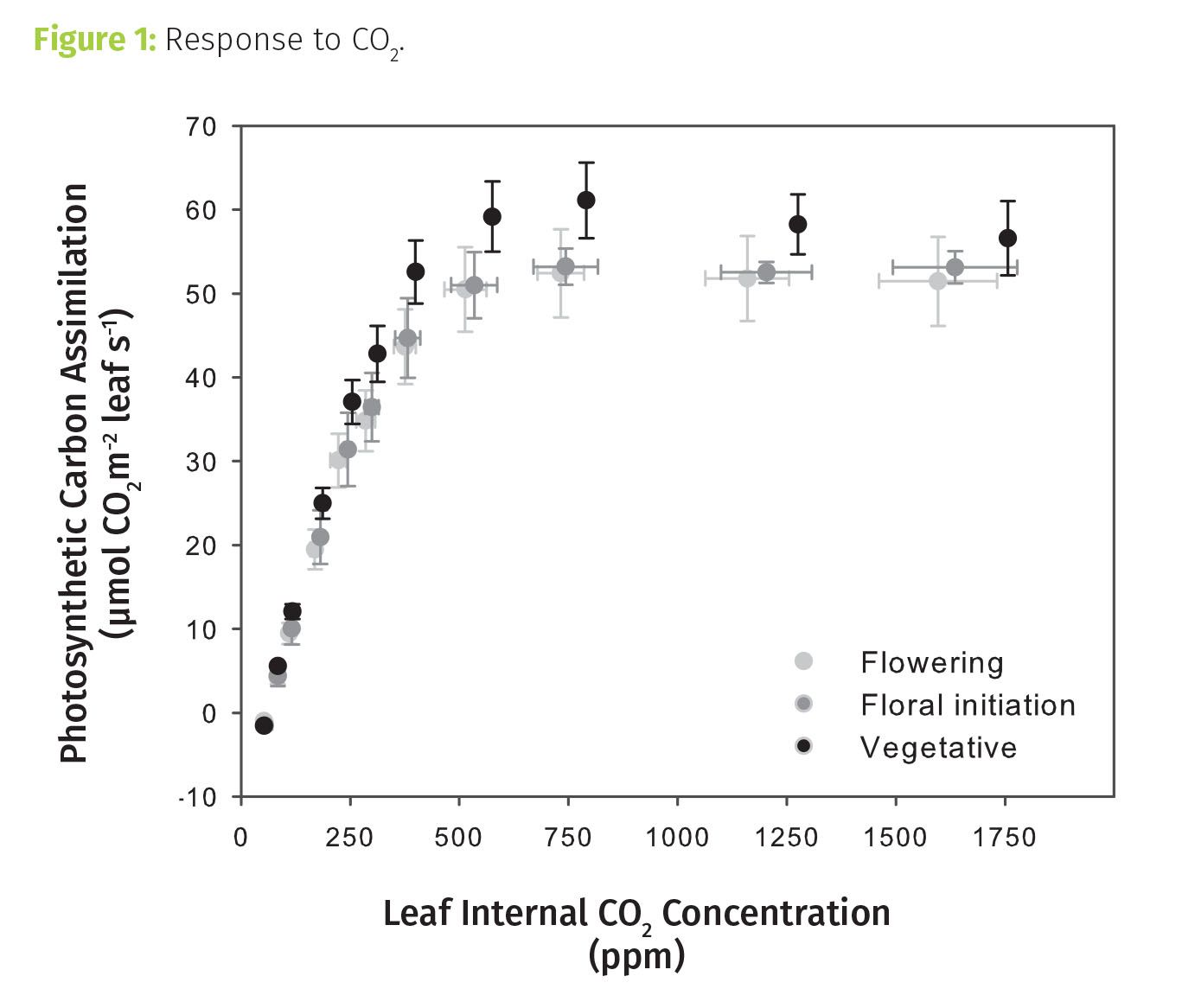
What does it mean when we don’t see that gradual increase at high CO2? From the lower CO2 concentration data for these plants, we might have expected the gradual rise to start around 750 ppm. Instead, the more mature plants in and approaching flowering showed near constant assimilation above that concentration. For the vegetative plants, assimilation decreased! In both cases, this is indicative of a phosphate limitation. Phosphate is needed to export the sugars produced from assimilating CO2 during photosynthesis, freeing up the system for the next round of CO2 to sugar conversion. Where phosphate is limiting, that export is inhibited, essentially causing a photosynthetic “traffic jam” which reduces assimilation.
Tip 1: Photosynthetic response to CO2 can provide information on enzymatic capacities and nutrient status. Each phase of the response has a well-defined biological meaning that may translate to production practice. For the plants measured here, for example, the phosphate limitation apparent at high CO2 likely has consequences for photosynthesis even at ambient CO2.
Response to Light
Much like for CO2, the general response to light is well defined (see Figure 2). At low light intensities, increasing light causes a comparable increase in assimilation. At some point, the light intensity begins to exceed what the plant can use, and assimilation reaches some maximum value. Beyond that, additional light is potentially detrimental.
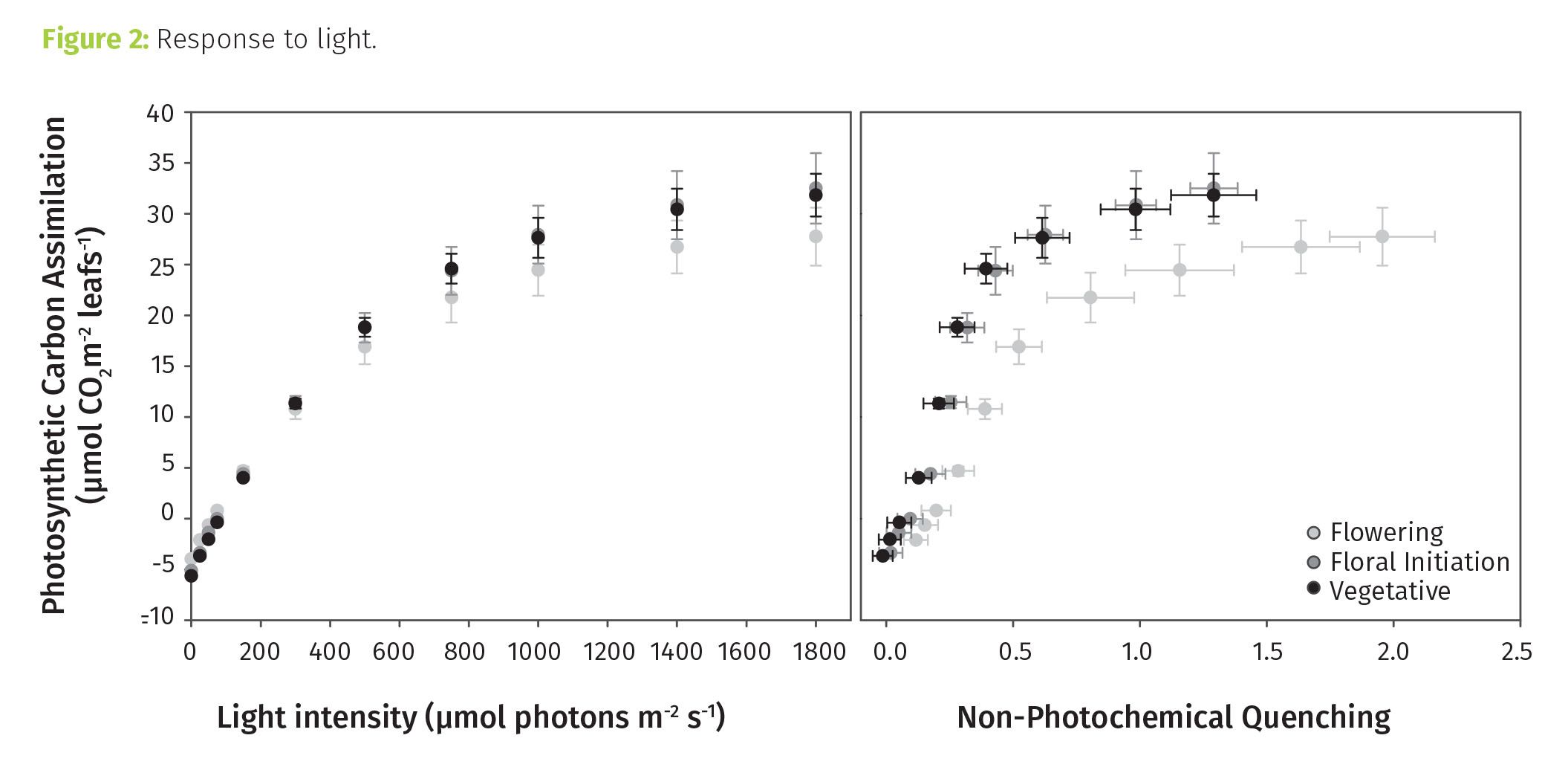
Nonphotochemical quenching (NPQ) gives insight to how light energy is being used. The higher the NPQ, the more light energy received by the plant that is being directed away from use in photosynthesis. It is a protective process and up regulated in response to several stressors.
Light response measurements showed that the early-stage plants had high photosynthetic capacity. As light intensities approached full sun intensity, assimilation continued to increase. What does this mean? “Janet’s G” in its vegetative form can use a lot of light, and given more, these plants would have done more with it. The same was not true for the plants in flower. They engaged more NPQ and at lower light intensities, suggesting they were less capable of dealing with light even at nominal ambient intensities.
Tip 2: Photosynthetic response to light can be used to make more creative, science-based decisions that may be particularly relevant when using artificial lighting. Certainly, more research is needed, and the findings will vary by variety, but think about what this dataset suggests: light could be reduced during flowering for “Janet’s G.” For indoor growers, this could equate to huge energy cost savings.
Plant Response Throughout the Day
Diurnal patterns in stomatal conductance suggested these plants would have been ill-equipped to deal with drought (see Figure 3). To take up CO2, plants must open their stomata and subsequently lose water. Stomatal conductance gives us a measure of stomatal opening and potential for water loss. Conductance is typically well coupled with assimilation, and for at least part of the day is generally expected to track light availability. Many plants show an afternoon depression in photosynthesis, which is typically accompanied by a reduction in conductance.
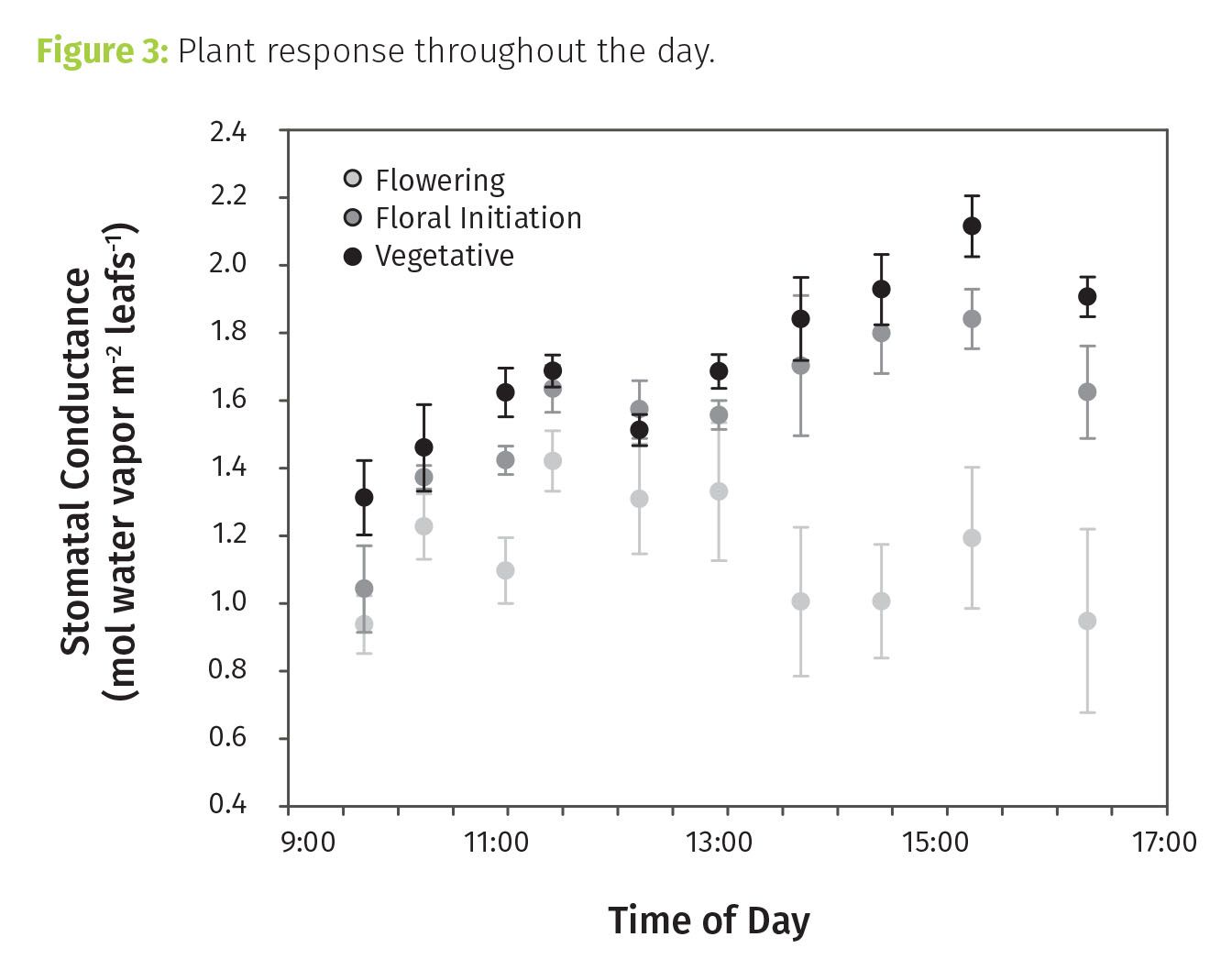
Only the flowering plants showed a mild afternoon depression. For early-stage plants, conductance was high and decoupled from photosynthesis in the afternoon. They were making no effort to conserve water. A sudden change in water availability would likely have had significant (detrimental) impacts.

Tip 3: Diurnal patterns can be indicative of stress sensitivities and onset of stress. Stomatal conductance, particularly, is linked to water status and can be a sensitive indicator of the onset of water stress, often well before wilting is evident.

Closing Remarks
Hopefully this gives some indication of the possibilities for this type of data. Fundamental physiological measurements like these underlie much agricultural and horticultural science and drive the products of those endeavors. There is open opportunity for application of these methods to cannabis. They hold promise for cultivation optimization and management, for development of the kinds of numerical tools used with other crop species, and for selection of physiologically robust lines. The latter point being critical for selecting varieties that not only yield what we want, but also can do it under the ever-changing environmental pressures where we grow them.
About the Authors
Jason Hupp, MS, is a Principal Scientist with LI-COR Environmental, specializing in the design, development, and application of leaf-level physiological instrumentation. Allison Justice, PhD, is the founder and CEO of The Hemp Mine.
How to Cite this Article:
A. Justice and J. Hupp, Cannabis Science and Technology® Vol. 5(7), 31-34 (2022).
