Can Analytical Testing Quality Be Assured in the Cannabis Industry?
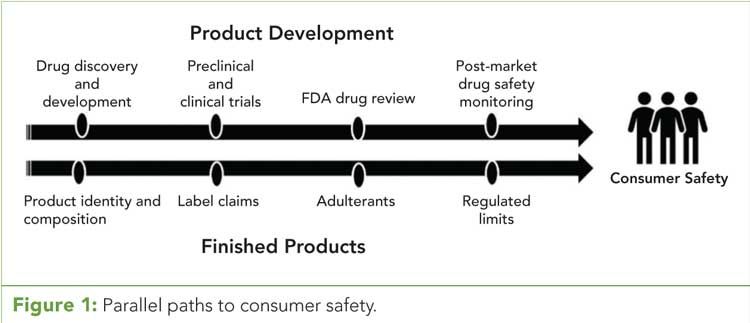
Figure 1 (click to enlarge): Parallel paths to consumer safety.
There is no dispute that cannabis is a complex plant often used as a botanical drug, food, food ingredient, and textile. With no federal oversight, regulatory bodies of the now 29 United States and the District of Columbia where cannabis is legal in some form, are tasked with ensuring product safety to their constituents. Most states require finished product testing by the International Organization for Standardization and the International Electrotechnical Commission (ISO/IEC) 17025 accredited laboratories. Laboratory accreditation to ISO/IEC 17025 represents a laboratory’s commitment to develop protocols to ensure quality practices are implemented and includes the attestation of their competence by an independent third party. Whenever possible or when mandated, analytical labs rely on published consensus and standard test methods or to validate their modifications to such methods to perform the necessary work. Although standard methods are in development by scientific organizations such as AOAC International and ASTM, as of this writing there is not a single published compendial or consensus method for the most typical assays used in the cannabis industry. This article discusses basic principles of quality assurance and laboratory accreditation, and the current status of voluntary methods in development. A final intent of this article is to provide unique insight into the challenges associated with testing cannabis amidst the federal prohibition.
The origins of cannabis appear to be rooted in Central Asia, with evidence of fiber use dating to 10,000 BC (1) and medicinal use dating to 3000 BC (2-4). For centuries, this robust plant has been purposed and repurposed in textiles, foods, medicines, and agricultural crop remediation around the globe (1,3-5). The medicinal utility of the plant eventually made its way to the West around 1839, largely from the clinical work and advocacy of William O’Shaughnessy (3,6). Recognizing the beneficial contributions in medicine, cannabis was formally introduced to the United States around 1850 and was included in the United States Pharmacopeia (USP) from 1851 to 1942 (7). Physicians and researchers supported the use of cannabis for myriad diseases and disorders. For example, in 1860 the Ohio State Medical Society’s Committee on Cannabis Indica reported efficacy of cannabis for treatment of stomach cramps, post-partum depression, and venereal diseases (1). Cannabis, however, wasn’t the only “medicinal” agent in use during the latter half of the 19th century—opium-containing products were commonplace for treatment of nearly any malady. With no requirement for labeling, products were marketed as “cure-alls” with the result being an accidental, or at least incidental, opium-addicted population (8). Although physicians recognized the deleterious effects, they were not able to contain or reverse the problem, thus leading the way for regulatory intervention.
In the interest of consumer safety, the US Congress enacted the Pure Foods and Drugs Act in 1906 (reorganized to the US Food and Drug Administration [FDA] in 1930) as a mechanism to ensure the quality and safety of food and drug products. Established laws required specific labeling for certain products known to contain designated “poisons” such as alcohol, morphine, and cannabis (9). The Harrison Act of 1914, which declared cannabis use illegal, and the Marihuana Tax Act of 1937, essentially banned cannabis from further medical use and halted relevant research (3). Federal controls over drugs and food were further tightened when the Pure Foods and Drugs Act was subsequently replaced by the Federal Food, Drug, and Cosmetic Act in 1938 (9). The Controlled Substances Act in 1970 established a classification system for certain drugs and maintained the earlier declarations of the dangers of cannabis. The ensuing drug scheduling system continues to classify a substance based on its potential for abuse and medical value. Since 1970 cannabis has been designated on the most restrictive level, Schedule I, implicating it as a substance with potential for abuse, and no medicinal value (11) and by federal law, illegal to possess or distribute. Even with the Schedule I classification of cannabis, science has been moving forward (and succeeding) to characterize cannabis and empirically elucidating potential contributions for its use in medical applications (12) including the identification of an endogenous cannabinoid system (13).
An insurgence against the federal prohibition of cannabis has been gaining momentum for more than three decades and shows no signs of slowing. As of this writing, 29 states in the United States plus the District of Columbia have sanctioned cannabis use for medically compromised or recreational (adult) consumers. A memo issued by Deputy Attorney General James M. Cole (also known as the “Cole Memo”) in 2013 provided guidance to indicate that federal enforcement agencies would be unlikely to prosecute “individuals with cancer or other serious illnesses who use marijuana as part of a recommended treatment regimen consistent with applicable state law” (14). This memo essentially positioned the governing states with responsibility to ensure consumer safety. Paradoxically, where states were one day prosecuting individuals for cannabis possession, they immediately became guardians and regulatory bodies for this federally illicit substance.
Defining Safety
For cannabis consumers, particularly those who are medically compromised, product safety is exceptionally important. Before we can discuss the merits of how to ensure safety, we must first define safety, as it can have different applications. Measures of safety are bifurcated along different, yet parallel paths (refer to Figure 1). (See upper right for Figure 1, click to enlarge.) Along the first path “safe” refers to, for example, the product’s effectiveness as a drug to treat medical specified conditions, determination of its toxicology, and opportunities to mitigate known contraindications. These elements are generally part of product or drug development schemes that typically require International Conference of Harmonization (ICH) quality guidelines (15), which are also subject to short and long-term monitoring programs. Along the second path, the regulation of safe products refers to assurance that products are correctly labeled and prohibitive additives or adulterants are absent. This “truth in labeling” was a prime intent of the Pure Foods and Drug Act of 1906 and is an element of the FDA’s Food Safety Modernization Act of 2011 (16). Although these two paths toward safety are parallel and critically important, they do not contradict each other and should be routinely implemented as part of good laboratory practices (GLP), good management practices (GMP), or good clinical practices (GCP) quality systems. These are typically referred to as “GxP” quality processes. GLP establishes a quality system framework used to support a laboratory research plan and performance of nonclinical health and safety studies in food, drug, biological products, and medical devices. These are regulations published in the Code of Federal Regulations, 21CFR part 58, and are enforced by the FDA (17). GMP, however, represent quality system requirements to ensure pharmaceutical products are consistently manufactured to product specification (18). Finally, GCP refers to a system of internationally accepted guidelines, also adopted by the FDA, for scientific quality standards relative to clinical studies involving human subjects (19).
Although the FDA has approved Marinol and Syndros, which are single-compound synthetic forms of Δ9-tetrahydrocannabinol, for certain medical conditions including nausea from chemotherapy and weight loss associated with AIDS (20), they have also clearly stated that there is no medical condition approved for the prescription of cannabis and there are no (botanical) cannabis or cannabis-derived drugs available to date (20). The FDA does, however, provide a pathway forward for cannabis drug development by supporting the Agricultural Act of 2014, which permits universities and state agriculture departments to cultivate cannabis for the explicit purpose to research its industrial potential (17). This paper refrains from discussion on cannabis drug development and the medical utility or medical safety of cannabis, and instead focuses on the characteristics of the raw or finished product.
Of the 29 states and the District of Columbia, all but five require some degree of laboratory analysis of finished products, including dried flower. Unlike other commodities such as food and feed, cannabis is afforded no federal regulatory oversight and hence, no regulated target values of analytes. Where cannabis use is legal, most regulatory divisions or jurisdictions require some degree of third-party testing ranging from minimal to exhaustive analytical profiles. Typical protocols include, for example, identification and quantitation of select cannabinoids, determination of solvent residues, qualitative or quantitative determination of mycotoxins, as well as the identification and quantitation of pesticide residues, quantitation of moisture, water activity, heavy metals analysis, identification of microorganisms, and various fungi. Product testing such as this is not new to analytical laboratories. In fact, many large-scale manufacturers (for example, food, feed, pharmaceutical, and tobacco) have quality assurance labs within their production facilities tasked to ensure that products are developed and manufactured according to specification before release for public distribution. In industries like pharmaceuticals and food, however, testing is required by a regulatory body like the FDA or the Food Safety and Inspection Service (FSIS) under the US Department of Agriculture to ensure products are accurately tested without bias, providing consumers confidence in their products.
Challenges to Ensuring Cannabis Product Safety
Laboratories testing cannabis and cannabis-infused products are challenged on several fronts. First and perhaps foremost, this complex (15) plant itself comprises nearly 500 compounds, at least 100 of which are cannabinoids, 120 terpenes, and dozens of flavonoids (22-24). By their very nature, many of the cannabis compounds are likely to interact with one another (15), requiring keen attention to component stabilities. Concentrations of constituents are inconsistent between structures within a single plant (such as flower, leaf, or stem) (22,25), which is further complicated when different cannabis strains are commingled and (theoretically) homogenized. This implies that sampling and subsampling are the most critical steps in testing. Further, cannabis plant material is prone to degradation and subsequent reactions (24), thus representing a dynamic and complex system where identification of single constituents is challenging at best, even under ideal conditions. Depending on the extraction technology used, the process may facilitate sample-product degradation. For example, cannabinoids are carboxylated in their natural state and may decarboxylate when processed in a mechanical grinder. Furthermore, Hazecamp and colleagues reported that some cannabinoids may be oxidized and isomerized while in the gas chromatography (GC) injector port and column, well before the sample reaches the oven (25).
The Lack of Certified Reference Standards for Laboratory Proficiency Tests
Analytical testing laboratories in most industries rely on the use of high-purity and well-characterized reference materials, preferably certified reference materials (CRM) accredited to the International Organization for Standardization and the International Electrotechnical Commission (ISO/IEC) 17034 (formerly ISO/IEC Guide 34) to validate analytical test methods. Currently there are more than a dozen cannabinoid CRMs available through several providers. The rigor of CRM formulation is susceptible, however, to timely and strict environmental conditions and processing to avoid in situ deterioration during manufacture, and then in the laboratory following purchase.
In most industries, third-party testing laboratories participate in commercially available proficiency test (PT) or interlaboratory comparison (ILC) programs as indicators of analytic competence. Many industries, including food and feed, purchase commercially available PT schemes that are accredited to ISO/IEC 17043, representing the most rigorous material characterization. Although there are differences between PT and ILC schemes, the intent of both is to provide a laboratory the opportunity to compare test results of a specified sample with the results of other participating laboratories. Presently, there are no accredited PT schemes available for the cannabis industry, nor is there an ILC program that utilizes a representative cannabis sample. Simply stated, this void exists because cannabis products cannot be legally shipped across state boundaries. Consequently, cannabis laboratories resort to purchasing commercial products that are essentially reference materials prepared at concentrations to accommodate legal transfer across state lines. With no method guidelines and no real extraction to perform, these samples are often referred to as “dilute and shoot” mixed proficiency samples. The resulting data represent an interlaboratory comparison of each laboratory’s skill at recovering the analytes of interest. It is noteworthy that there is no cannabis plant reference material with well characterized analytes and no clean surrogate that could be enhanced with cannabinoid CRMs to provide a realistic determination of laboratory proficiency.
Laboratory Accreditation
Many of the states that require cannabis-product testing by third-party laboratories also require that laboratories be accredited to ISO/IEC 17025 by a recognized accreditation body (AB). ISO/IEC 17025 is the pinnacle international standard of accreditation for laboratory quality and technical competence. The accreditation process relies on a uniform approach of technical assessment, shown to increase confidence in data through traceability and reproducibility (36). Accreditation bodies are themselves accredited to ISO/IEC 17011 and subject to peer evaluations in the cooperative. Popular ABs for the United States cannabis industry are the American Association for Laboratory Accreditation (A2LA), ANSI-ASQ National Accreditation Board (ANAB), and Perry Johnson Laboratory Accreditation (PJLA). The fundamental difference between a certification program such as ISO/IEC 9001 and ISO/IEC 17025 is that 17025 accreditation is the determination of technical competence to perform specified methods by an independent and impartial third party, whereas ISO/IEC 9001 certification means a laboratory has a quality management system. Governments and regulatory bodies worldwide have embraced ISO/IEC 17025 as a means to establish confidence that the laboratory product (derived data) is quality driven.
Standard and Consensus Test Methods: What Is the Difference?
Prescribed practices, including analytical laboratory test methods, can be developed by any person, laboratory, or organization and may be promoted as “standard practices.” Before implementing a standard of any sort, however, users should thoroughly investigate and appraise the basis of the standard practice and also evaluate the history and credibility of the person or organization that developed the standard. In terms of standard analytical test methods, once a method is published in a scientific journal, it may be adopted by any laboratory and referenced as a published method. The term consensus methods, however, refers to methods that have undergone an extensive peer-review process, through which participants have reached consensus on the utility and specification of a given method. Typically, as consensus methods become preferred or required by a governing body, they are referenced as “standard methods.”
One of the more technical requirements of ISO/IEC 17025 is the application of analytical methods that are scientifically sound. Most often, laboratories will choose standard or consensus test methods that have been peer-reviewed where major performance parameters including measurement uncertainty have been specified. Laboratories adopting these standard methods must demonstrate the ability to properly implement the methods before introducing actual test samples. On occasion, a laboratory may choose to modify an existing standard method. In this case, the laboratory must demonstrate equivalency of the modified method to the standard method. This process is referred to as method verification. In the absence of standard methods, laboratories are compelled to develop and validate their own methods. ISO/IEC 8402 defines method validation as “the confirmation by examination and provision of objective evidence that the particular requirements for a specific intended use are fulfilled” (27). AOAC International, ASTM, and the American Chemical Society (ACS) have published guidelines for single laboratory method validation. In general, method validation studies must demonstrate statistically sound performance with respect to precision, accuracy, specificity, linearity, selectivity, detection limit, limit of quantitation, and so on, and must also include measurements of uncertainty (28,29).
Currently, there are no standard test methods available for dried cannabis flower or any other cannabis matrix. Several scientific organizations have embraced stakeholder needs and are actively developing standard methods: AOAC International, American Oil Chemist Society (AOCS), ASTM International, and the US Pharmacopeia (USP). Each of these institutions carries a long and rich service history of developing standards as elements to the provision of consumer safety. The AOAC was initially formed under the US Department of Agriculture in 1884 as the Association of Agricultural Chemists, and through the years became an independent and international organization encompassing all branches of science that affect (predominantly) the food and feed industries. Many of the voluntary methods approved by the AOAC’s Official Method Board are recognized as industry standards by the FDA and other regulatory bodies (27). Similarly, ASTM International was established in 1898 to address stakeholder needs for standard specifications for materials. Since its inception, ASTM formed many communities designed to identify and address their specific needs. Although developed methods are intended to be available for voluntary use by stakeholders, they often become recognized as industry standards (31). Formed as the Society of Cotton Products Analysts 1909, the AOCS has been providing voluntary methods for the oil and fat communities (32). Finally, the USP was established in 1820 to provide a formulary of medicines to ensure public safety. Over the years, they have developed standards for food ingredients, medicines, and dietary supplements (33). As with the other organizations, the USP is not a regulatory body, but their standards are enforceable by the FDA.
In 2016, the AOAC International formed a Cannabis Advisory Panel and Cannabis Working Group. They have since developed several standard method performance requirements (SMPRs) for analytical methods, and an Expert Review Panel is currently evaluating candidate methods. In 2017 the ASTM formed Cannabis Division, D37, that has identified subcommittees to address the broad spectrum of stakeholder needs such as personnel, cultivation, laboratory testing, process and handling, and security and transportation. Also in 2017, D37 hosted a cannabis and hemp workshop in Berlin to engage with European stakeholders. The AOCS has also embraced the opportunity and are working with stakeholders to develop standard test methods. Establishing and vetting these methods is neither easy nor quick; however, each organization is diligently at work. Once established, most consensus or standard test methods endure for decades because of their sound and solid scientific foundations.
Summary and Outlook
In summary, cannabis has been around for at least 5000 years and much of its history has been held in high regard for its many uses in rituals, medicines, textiles, and personal pleasure. However, for the past 120 years or so, the pendulum has swung from open acceptance to complete prohibition. The past decade has witnessed this shift once again, where in the United States there remains a federal prohibition, but more than half of the United States has legalized some form of cannabis use. Effectively, the United States cannabis industry is subject to 30 independent operating systems managed by jurisdictions or state governments that historically prosecuted the industry. Where consumers of similar commodities are afforded protection by one or more federal body, individual states are tasked with this challenge and unfortunately not all of them have recognized the necessity for product testing. Admittedly, cannabis product testing is not always straightforward. For example, an analytical method to extract, identify, and quantitate cannabinoids in a chocolate bar is not likely to perform similarly in a chewing gum matrix.
The inherent complexities of the cannabis plant, the vast number of matrices in which it may present, and the number of modes by which it can enter a person’s body creates a dynamic analytical challenge. Assuring quality in cannabis testing requires simultaneous movement along multiple pathways, beginning with consistent regulatory requirements. States that have legalized cannabis use have a responsibility to their constituents to ensure products are as safe as possible, and should honor that commitment by providing the necessary resources to relevant organizations. This begins with quality manufacturing as well as quality testing of finished products by third-party testing laboratories. Laboratories should be compelled to operate transparently and competently, as determined by an independent third party and not by self-declaration. Laboratory accreditation to ISO/IEC 17025 attests to quality assurance. Quality control, however, is the responsibility of laboratories. Among other factors, effective quality control requires access to well-characterized and standardized materials that are not limited by state boundaries. Organizations such as AOAC, AOCS, USP, and ASTM are working with scientists and stakeholder communities to establish consensus methods built upon sound science as one element of consumer protection. In the end, however, ensuring the safety of cannabis products requires a host of conditions that have yet to be met.
Susan Audino, PhD, is the Principal at S.A. Audino & Associates, LLC. She is a chair for the AOAC International Cannabis Advisory Panel, a chair for AOAC International Cannabis Working Group, an executive committee member for ASTM D37, and the lead assessor and instructor for A2LA. Direct correspondence to: susan.audino@gmail.com
References:
- M. Booth, Cannabis: A History (St. Martin’s Press, New York, 2005).
- E. Russo, H.E. Jiang, X. Li, A. Sutton, A. Carboni, F. del Bianco, G. Mandolino, D.J. Potter, Y. Zhao, S. Bera, Y. Zhang, E. Lu, D.K. Ferguson, F. Hueber, L. Zhao, C. Liu, Y. Wang, and C. Li, J. Exp. Bot. 59(15), 4171-4182 (2008).
- L.L. Iversen, The Science of Marijuana (Oxford University Press, New York, 2008).
- E.B. Russo, in Handbook of Cannabis, R.G. Pertwee, Ed. (Oxford University Press, New York, 2014), pp. 23-43.
- V. Angelova, R. Ivanova, V. Delibaltova, and K. Ivanov, Industrial Crops and Products 19(3), 197-205 (2004).
- S. Mukherjee, The Public Domain Review, Retrieved January 15, 2018 from https://publicdomainreview.org/2017/04/19.
- US Pharmacopeia, 3rd Edition. Retrieved January 15, 2018, from http:antiquecannabisbook.com/view.timeline.php.
- D.F. Musto, Scientific American 20-27 (July 1991). Retrieved from https://faculty.unlv.edu.
- Federal Food and Drugs Act of 1906 (The “Wiley Act”), Public Law Number 59-384, 34 STAT.768 (1906), 21 USC Sec 1-15 (1934). Retrieved from www.fda.gov/regulatoryinformation/lawsenforcedbyFDA/ucm148690.
- H.R. 18583, 91st Congress of the United States (1970).
- United States Drug Enforcement Administration. Drug Scheduling. Retrieved from www.dea.gov/druginfo/ds.
- National Academies of Sciences, Engineering, and Medicine, The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research (The National Academies of Press, Washington, DC, 2017). Doi:10.17226/24625.
- V. DiMarzo, D. Melck, T. Bisogno, and L. De Petrocellis, Trends in Neurosciences 21, 521-528 (1998).
- J.M. Cole, Deputy Attorney General. Memorandum for all United States Attorneys (August 29, 2013). Retrieved January 10, 2018 from www.mpp.org/federal/cole-memo.
- B.T. Yeh, The Controlled Substances Act: Regulatory Requirements (2012). Retrieved January 15, 2018 from https://fas.org/sgp/crc/mis.
- Federal Drug Administration Food Safety Modernization Act of 2011. Retrieved January 15, 2018 from https://www.fda.gov/food/guidanceregulations/fsma.
- US Food and Drug Administration, Good Laboratory Practices. Retrieved January 30, 2018: www.fda-glp.com (FDA, Rockville, Maryland).
- US Food and Drug Administration, Facts about Current Good Manufacturing Practices. Retrieved January 30, 2018: www.fda.gov/drugs/development/approvalprocess/manufacturing (FDA, Rockville, Maryland).
- International Conference on Harmonization, Harmonised Tripartite Guideline, Guideline for Good Clinical Practice, E6(R1) (ICH, Geneva, Switzerland, 1996).
- US Food and Drug Administration, FDA and Marijuana: Questions and Answers. Retrieved January 30, 2018: www.fda.gov/newsevents/publichealthfocus (FDA, Rockville, Maryland).
- H.R. 2642, 130th Congress of the United States, 2nd Session (2014).
- M.A. ElSohly, and D. Slade, Life Sciences 78, 539-548 (2005).
- G.A. Cabral, E.S. Raborn, and G.A. Ferreira, “Phytocannabinoids and the Immune System,” in Handbook of Cannabis, R.G. Pertwee, Ed. (Oxford University Press, New York, 2014), pp. 261-274.
- U. Pagotto, G. Marsicano, D. Cota, B. Lutz, and R. Pasquali, Endocrine Reviews 27(1), 73-100 (2006).
- M.A. ElSohly, in Emerging Issues in Analytical Chemistry, B.F. Thomas, Ed. (Elsevier, Amsterdam, The Netherlands, 2016).
- A. Thompson and V. Langfield, in Handbook of Cannabis, R.G. Pertwee, Ed. (Oxford University Press, New York, 2014), pp. 356-372.
- M. Backes, Cannabis Pharmacy (Black Dog & Leventhal Publishers, New York, 2014).
- M. Starks, Marijuana Chemistry (Ronin Publishing, Inc., Berkeley, California, 1990).
- A. Hazecamp, A. Peltenburg, R. Verpoorte, and C. Giroud, Journal of Liquid Chromatography & Related Technologies 28, 2361-2382 (2005).
- International Laboratory Accreditation Cooperation (ILAC). Retrieved January 20, 2018 from www.ILAC.gov.
- International Organization for Standardization. (1994). Quality management and quality assurance - vocabulary (ISO Standard No. 8402).
- E. Pritchard and V. Barwick, Quality Assurance in Analytical Chemistry (Wiley Publishers, West Sussex, UK, 2007).
- M.E. Swartz and I.S. Krull, Handbook of Analytical Validation (CRC Press, Boca Raton, Florida, 2012).
- AOAC International. Retrieved January 29, 2018: www.aoac.org/aoac_prod_imis/APAC/AB/AOAC.
- ASTM International, The History of ASTM International. Retrieved January 29, 2018: www.astm.org/about/history_book.
- American Oil Chemists’ Society, History of AOCS. Retrieved January 29, 2018: www.aocs.org/info/about-aocs/history-of-aocs.
- US Pharmacopeia, Our History. Retrieved January 29, 2018: www.usp.org/about.
How to Cite This Article
S. Audino, Cannabis Science and Technology 1(1), 14-20 (2018).

Insights on Cannabis Testing Challenges and Industry Standards: An Interview with Douglas Duncan
August 9th 2024In light of recent headlines concerning cannabis laboratories throughout the country, Cannabis Science and Technology reached out to Douglas Duncan, Laboratory Director of Kairos Labs in Detroit, MI and member of our Editorial Advisory Board for more information. In this interview, Duncan shares his perspectives on lab shopping, major challenges in the industry today, and innovations in cannabis testing laboratories for the future. He also shares insights into consumer practices and the potential effects of a federal rescheduling of cannabis.