Using Method Detection Limits to Set Mandatory Reporting Limits for Banned Pesticides: How a Public-Private Partnership Improved Cannabis Regulation in Colorado
Image credit: Kiattipong/shutterstock.com

The discovery of nonregistered pesticides contamination in cannabis plant material in Colorado prompted the need to set mandatory reporting limits (MRLs) for these pesticides in the state to ensure public safety. Since then, the Colorado Department of Public Health and Environment (CDPHE) undertook the responsibility for setting such MRLs. Colorado’s novel approach to determine MRLs for each pesticide involved forming a working group with its in-state laboratories and expert volunteers to agree on analytical procedures to determine method detection limits (MDLs) using an industry-standard approach. The authors describe how this approach formed a collaborative public-private partnership where state government agencies worked side by side with laboratories and growers toward a common goal. The multilaboratory study was completed yielding MDLs for 13 pesticides in cannabis flowers using a QuEChERS (quick, easy, cheap, effective, rugged, and safe) extraction with analysis by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The authors share the procedures used for the study, an overview of the data generated by the participating laboratories, and the new Colorado MRLs, which became effective on January 1, 2018.
The regulation of pesticide use on cannabis is a complex issue for states with legalized marijuana. Pesticide use is regulated at the federal level; however, cannabis is not a specifically listed crop on any registered pesticide label, and the United States Environmental Protection Agency (US EPA) does not consider cannabis to fit into any general crop groups, such as herbs or spices (1). In addition, no risk assessments have been conducted specifically for pesticide use on cannabis and tolerance limits have not been defined. Pesticide use in cannabis was recognized as a public health and safety concern, but the information deficit and limited available resources left the state of Colorado grappling with how to handle this complex issue when the Denver Department of Environmental Health placed the first holds on 100,000 marijuana plants for improper use of banned pesticides in March 2015.
In the following months, many additional quarantine and recall events occurred. In November 2015, Colorado issued a statewide pesticide policy and executive order (2) addressing the use of pesticides in marijuana. These documents mandated state agencies to consider cannabis contaminated with banned pesticides a threat to public safety, until such a time that scientific assessment establishes which additional pesticides can be safely applied to marijuana.
After issuance of the executive order and statewide policy, the next question was how to get private laboratories certified to perform pesticide testing so the state could mandate this testing for cannabis. Two primary pieces of information were needed: what pesticides are laboratories expected to test for and what are the associated limits? The executive order has frequently been called a zero tolerance policy; while it does establish zero tolerance for the use of a banned pesticide, analytically it is not possible to test to a concentration of zero. Without defining minimum testing requirements, different results would be reported across laboratories. To address these issues, the state initiated a stakeholder process to determine what pesticides should be tested for and what the associated mandatory reporting limits (MRLs) should be.
The Colorado Department of Public Health and Environment (CDPHE) took the lead on oversight of this stakeholder process, which engaged marijuana testing facilities, cultivators, product manufacturers, scientific experts, and other interested parties. The working group first tackled the list of required analytes. The final list selected included 13 pesticides that were considered the most important for testing in Colorado because they are banned for use and have been previously detected in marijuana in the state.
The working group then determined that in the absence of established pesticide tolerance limits, the regulatory limits should be based upon laboratory detection capability. At that time, to obtain this information, a unique public-private collaboration was initiated to design and execute a multilaboratory method detection limit (MDL) study engaging the resources of Colorado’s private marijuana testing facilities and the state’s Department of Agriculture (CDA) laboratory. The results of the study would be used to inform the regulatory limits for the list of 13 pesticides in marijuana. A technical subcommittee of scientists was formed from the stakeholder working group to assist CDPHE in designing the study and reviewing the results.
Study Design and Preparation
Following the decision to design a multilaboratory study (MLS), Restek volunteered to help CDPHE form a study design and oversight (SDO) team to ensure the multilaboratory study’s success. Since no study of this kind had ever been attempted in the past and there was no standardized analytical method protocol for participant laboratories to follow, the SDO team’s goal was to minimize variables by applying study guidelines based on industry-standard practices. Also, since the marijuana flower buds are an expensive commodity and time was of the essence given the governor’s directive, careful planning and execution were required to produce statistically valid data that the state could use for decision-making upon completion of the first round.
The team consisted of two CDPHE personnel, one representative from Neptune and Company, one from Lowry Consulting-all based in Colorado-and three from Restek Corporation located in Bellefonte, Pennsylvania. The team began to meet regularly to design guidance documents by which labs would be asked to conduct their testing.
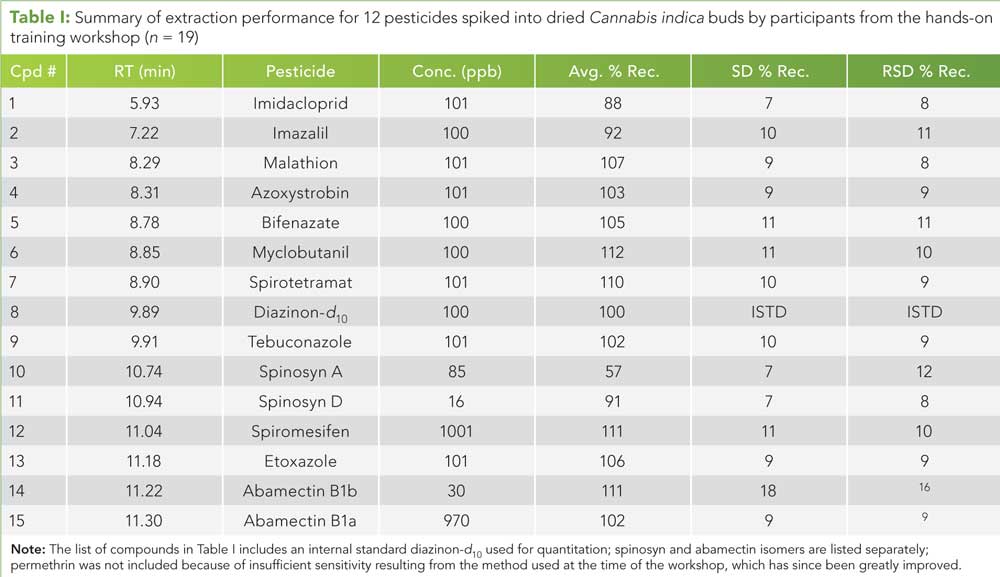
Table I (click to enlarge): Summary of extraction performance for 12 pesticides spiked into dried Cannabis indica buds by participants from the hands-on training workshop (n = 19)
The overall study was designed to progress through three sequential phases one built upon another, culminating with participating laboratories completing all of the steps toward reporting MDLs for pesticides in marijuana buds. These steps included determining an instrument detection limit (IDL), matrix quality control check (MQC), and a formal MDL. Three documents were constructed by the SDO team to guide laboratories through the MLS process followed by the technical subcommittee review, which included the volunteer labs. The technical subcommittee decided that liquid chromatography-tandem mass spectrometry (LC-MS/MS) would be the required analytical technique for the analysis to produce the best MDL data.
The first document described the steps to be taken by each laboratory to establish IDLs for each compound on their LC-MS/MS instrument. While some laboratories had already established such limits, this procedure was important as a starting point since some laboratories had either just purchased their instrument, or had not already completed this step in their laboratory. It was also necessary to establish whether the participant laboratories would be able to detect all 13 compounds at similar levels before spiking into actual matrix.
The second document, an MQC procedure, described steps to be taken by each laboratory to spike all 13 pesticides into cannabis flower extracts at varying concentration levels, then analyze eight replicates at each level to observe detectable levels they could achieve given acceptance criteria before executing the MDL study. The third document, the MDL procedure, described steps laboratories would follow to spike a dried flower matrix, verified to be free of the analytes of interest, then take it all the way through extraction, cleanup, and analysis. It is important to note that all three documents incorporated guidelines established by the SANTE 2015 Guidance document on analytical quality control and method validation procedures for pesticides residues analysis in food and feed (3). Such guidelines included transition ion ratio and calibration acceptance criteria. The MDL document incorporated the procedure from the Code of Federal Regulations 40 CFR Appendix B to Part 136 (4).
Other steps taken by the SDO team and stakeholder working group in Colorado included further minimizing variables, requiring all participating laboratories to use the same reference standards from a single source and a composite sample consisting of cannabis buds donated by a grower in Colorado. Restek donated two reference standards mixes and OrganaLabs donated the Cannabis buds. The Colorado Department of Agriculture (CDA) prepared extracts and dried plant composite materials to the study laboratories by grinding, compositing, and extracting throughout the various phases of the study.
The SDO team decided that at each of the three phases of the study laboratories would submit their results in spreadsheets provided along with each study document. The results were accompanied by raw data components as outlined in each of the three documents. All data submitted were reviewed by the SDO team to maintain confidentiality of the study. It was also decided, after completion of the IDL phase, that a nondisclosure agreement would be signed by the SDO team members to protect any proprietary information laboratories might share during the course of the study. This step contributed to a two- to three-month delay in the process before launching into phase two.
Before launching the MLS, training was offered to laboratory personnel and the CDPHE to ensure that participants were aligned on a general procedure in the absence of a published test method. The training was coordinated by Restek and Shimadzu personnel, who designed a one-day hands-on pesticides sample preparation and analysis workshop. CDPHE hosted the event, providing laboratory space and safety equipment for participants. Three suppliers contributed equipment, consumables, instrumentation, and personnel free of charge to the study group and CDPHE. Spex Sample Prep loaned its grinder for sample processing, Shimadzu Scientific Instruments loaned its LC-MS/MS instrument and personnel for extract analyses, and Restek donated reference standards, sample preparation consumables, and personnel time. Restek and Shimadzu instructors led 19 participants through the steps of a hands-on QuEChERS (quick, easy, cheap, effective, rugged, and safe) pesticides extraction with dispersive solid-phase extraction (dSPE) clean-up and filtration, followed by instrument setup and analysis of extracts by LC-MS/MS. Each participant prepared his or her own sample extract from donated dried Cannabis indica buds, spiking 11 pesticides at 100 ppb and two pesticides at 1000 ppb into 1.5-g samples. The combined results of the 19 extracts are shown in Table I. (See upper right for Table I, click to enlarge.)
Before the study was launched, it was decided that the participant laboratories would follow their own internal method procedures for the extraction and analysis of pesticides in a cannabis bud matrix, and incorporate what they had learned from the training as necessary. A 3.0-g sample would be utilized by CDA to create the composite extracts for the MQC study phase. Participant laboratories used between 1.5 g to 2.0 g of sample for the MDL phase because the actual amount varied from one laboratory to another based on their internal procedures.
Timeline of Study Events
The initial stakeholder groups were formed in January 2016 to address the Executive Order 2017-015 (1) and implementation of pesticide testing. The group spent several months gathering information concerning the determination of pesticide limits from other state programs, comparing regulatory limits, and evaluating the best scientific approach before the decision was made to work with the laboratory community as a whole on establishing pesticide MRLs.
The study design, organization, and initial recruitment of laboratories to participate was completed by July 2016. Although nine laboratories volunteered to participate in the study, MDL data from eight laboratories would eventually be received by the close of the study. The IDL study was initiated in mid-July 2016 with data delivery reported in August; however, the data submittal timeline was extended to laboratories that required additional time. The data for the IDL studies were compiled and released in November 2016 followed by the MQC phase, which began with the release of study samples in January 2017. After the MQC study data were gathered and processed, it was released in April 2017. The final step was for the laboratories to generate their MDLs using homogenized dried bud samples, which were provided to the laboratories in June 2017 with a study close date of July 2017. The data submitted by eight volunteer laboratories were statistically evaluated and reviewed, then released to the participating laboratories, CDPHE, and CDA in September 2017. From start to finish, the entire study took less than 20 months to complete.
Figure 1 (click to enlarge): Pesticides MDL data summary for the eight volunteer laboratories.
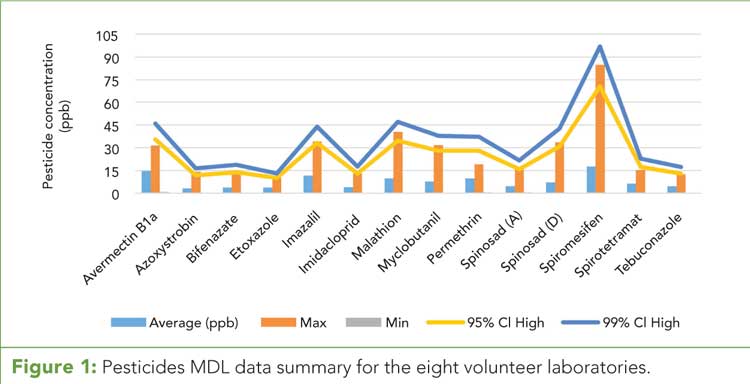
Figure 2 (click to enlarge): Percent relative standard deviations (%RSDs) by compound among participant laboratories.
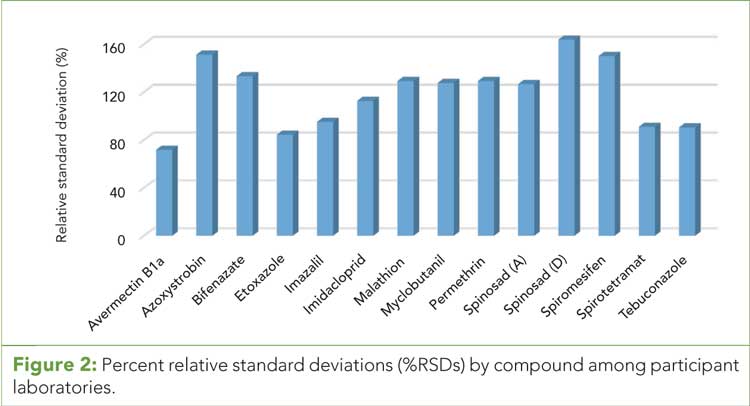
Figure 3 (click to enlarge): Pesticide average MDLs with 99% confidence interval.
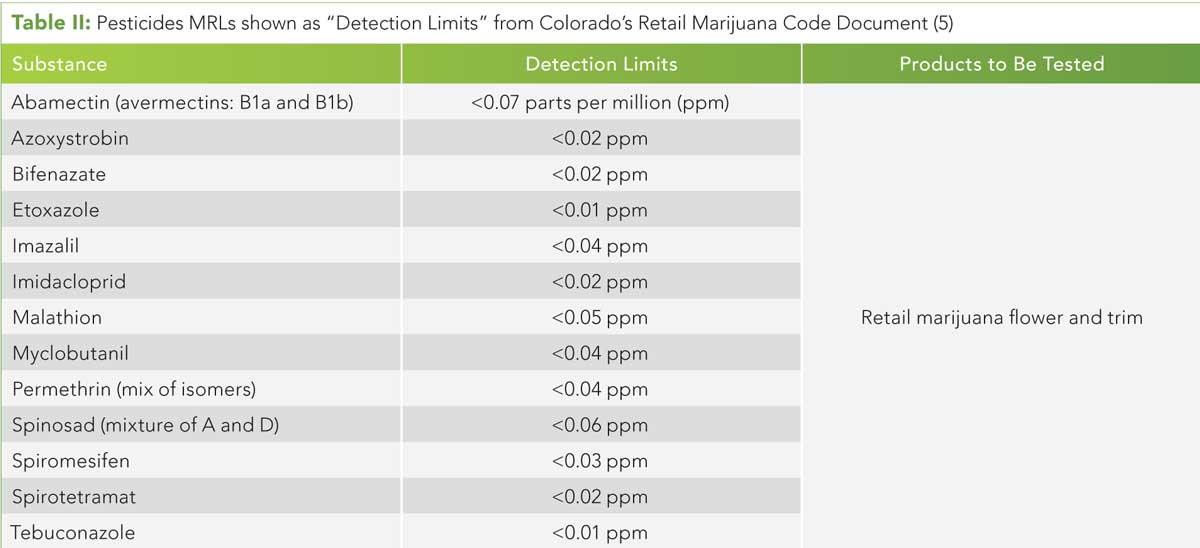
Table II (click to enlarge): Pesticides MRL s shown as “Detection Limits” from Colorado’s Retail Marijuana Code Document (5)
Study Data Analysis
The reported data collected across all of the analytes consisted of a small number of results for each analyte. The removal of any outliers would have had a large impact on the data. As a consequence, only one data point was removed as an outlier for the analyte permethrin. The data point was removed because it was an order of magnitude greater than any of the other data reported for permethrin.
Basic robust statistics were calculated: the mean, median, variance, standard deviation, percent relative standard deviation, and the upper limits 95% and 99% confidence intervals.
Figure 1 displays how the data compare across the analyte list for average MDL, minimum and maximum values, and the confidence intervals. (See upper right for Figure 1, click to enlarge.) The data for spiromesifen and malathion demonstrate the greatest amount of variability for the analytes in the study. Though not surprising, the extent of the variability was not expected. Removing spiromesifen, the remaining analytes fall into two categories, analytes below 30 ppb and 15 ppb, which is similar to MDL values found in other complex plant matrices.
Figure 2 shows relative standard deviations (%RSDs) for the eight volunteer laboratories for all analytes and represents several key factors that are being addressed by the industry: The lack of reference methods, inexperience with this complex analysis, and an extremely complex interfering matrix. (See upper right for Figure 2, click to enlarge.) The SDO team recognized these factors as key influencers affecting the higher %RSD values.
The SDO team recommendation was for the state to adopt the 99% confidence interval as the initial MRL for each analyte. This recommendation would incorporate the capabilities of the current laboratory community into the MRL determination, ensuring that the laboratories would at least be able to detect the analytes of interest.
The CDPHE was tasked with determining the MRLs for the pesticides based on the data and recommendation of the SDO. CDPHE staff including toxicologists, statisticians, marijuana laboratory certification staff, and analytical scientists were gathered. This group reviewed known toxicological data and other state pesticide MRL information to set the recommended MRL. They developed recommended MRLs for each pesticide and these were presented to the stakeholder group. After receiving public comment and feedback, the information was collected and the MRLs at the 99% confidence interval were incorporated into draft rules for consideration in official rule-making procedures.
Conclusions and Recommendations
The state of Colorado announced the rule-making process was completed and Rule 712 was adopted on November 17, 2017 and became effective January 1, 2018. The new rule, in addition to other contaminants, includes the 13 pesticides now required for testing and the MRLs are based on the MDLs from this multilaboratory study (4). See Table II for a summary of the MRLs. (See upper right for Table II, click to enlarge.) The approach of incorporating MDLs to set MRLs was challenged by members of the analytical community in Colorado who cited that they were not risk-based. However, regulators in Colorado persisted, explaining that the approach was sound given that the MRLs are not meant to be risk-based limits, but are instead set to ensure laboratories can detect banned pesticides that are not permitted for use in Colorado with acceptable precision, accuracy, and sensitivity from a cannabis bud matrix.
A number of noteworthy achievements have resulted from this effort. It is important to recognize this is the first and only study of its kind in the cannabis industry. This effort demonstrated that public-private partnerships can successfully resolve challenges while saving time and money through volunteer effort. It also demonstrated that when such efforts are undertaken with appropriate leadership, experience, and planning, the timeline for completion is shortened. Taking less than 20 months to complete a study of this magnitude is generally considered astoundingly fast in the science community. In addition, using a science-based approach to setting regulatory levels for contaminants in cannabis buds to protect public health is also a novel and uncommon approach among rule-making practices. Its successful application in this case demonstrates a viable approach that might be applied to similar challenges in other states as cannabis regulations emerge and develop. Finally, observing government and industry working together as a stakeholder community, volunteering time, equipment, materials, and space to solve a problem is not only encouraging, but shows that when given proper definition of a problem accompanied by a solution the community will rise to the challenge and deliver beyond expectations.
The study represents a public-private partnership among state and local government agencies with the marijuana industry, testing laboratories, cultivators, extraction laboratories, and infused product manufacturers. The goal was to provide a scientific, fact-based recommendation for the determination of pesticides in marijuana MRLs. As we noted with regard to the large variability of the %RSDs, the industry will benefit from standardized test methods, and greater experience. The process should be reviewed in the future once laboratories have developed improved methods and gain additional experience. The MDL procedures for each laboratory should be completed annually. A review of these collected MDL studies would yield a good potential metric to repeat the study. This will allow the MRLs to be adjusted to the current laboratory competencies.
Acknowledgments
The authors wish to thank the following individuals, government agencies, and companies for their significant contributions to the success of this collaborative effort: Eric Petty, CDPHE; Julie Kowalski, Trace Analytics; Jeff Lowry, Lowry Consulting; Jack Cochran, VUV Analytics; Jeff Dahl, Shimadzu Scientific Instruments. The Colorado volunteer cannabis testing laboratories: Gobi Labs, TEQ Analytical Laboratories, RM3 Labs, Nordic Analytical Laboratories, Agricor Laboratories, Aurum Labs, and CDA’s Laboratory. The Colorado Department of Revenue, The Colorado Department of Agriculture, The Colorado Department of Public Health & Environment, Neptune and Company, Organa Labs, Spex Sample Prep, Shimadzu Scientific Instruments, and Restek Corporation.
Joseph Konschnik is with Restek Corporation in Bellefonte, Pennsylvania. Heather Krug is with the Colorado Department of Public Health & Environment (CDPHE) in Denver, Colorado. Shawn Kassner is with Neptune and Company, Inc., in Lakewood, Colorado. Direct correspondence to: joe.konschnik@restek.com
References:
- Colorado Department of Agriculture, Factual and Policy Issues Related to the Use of Pesticides on Cannabis, (paragraph 4), CDA link: https://drive.google.com/file/d/1V_zVKP9R8qpdgYJ2r OrJR39b6MA1M9AH/view.
- Office of the Governor, Denver, Colorado, D2015-015 Executive Order Directing State Agencies to Address Threats to Public Safety Posed by Marijuana Contaminated by Pesticide (November 12, 2015).
- European Commission Directorate-General For Health and Food Safety, SANTE 2015 Guidance document on analytical quality control and method validation procedures for pesticides residues analysis in food and feed (SANTE/11945/2015 30 November-1 December 2015 rev. 0).
- Code of Federal Regulations (CFR), 40 Appendix B to Part 136 -Definition and Procedure for the Determination of the Method Detection Limit-Revision 1.11. (J U.S. Government Printing Office, Washington, D.C., 2011).
- Colorado Retail Marijuana Code Adopted Rules, CCR 1 212-2, Rule 712, Section R 712 - Retail Marijuana Testing Facilities: Sampling and Testing Program, E. Permissible Levels of Contaminants, (Adopted November 17,2017; effective January 1, 2018), pp. 124-125.
How to Cite This Article
J. Konschnik, H. Krug, S. Kassner, Cannabis Science and Technology1(1), 42-47 (2018).

The Cost of Compliance in the Cannabis Industry
April 1st 2025The financial and reputational risks of non-compliance far outweigh the upfront investment required to establish robust compliance systems. This blog explores the true cost of compliance, what non-compliance can mean for a business, and why investing in compliance is a proactive, strategic decision rather than a burdensome expense.