Looking with Light: Understanding Gas Chromatography, Part II: Sample Introduction and Inlets
Understand the function and chemistry of GC systems and how to adapt that function and methodology to our own analyses.
Gas chromatography (GC) is an important part of an analytical laboratory which has a long history of robust function. In the past decades, the technology has improved and changed to accommodate a wide variety of compounds and targets. In this edition of “Navigating the Labyrinth," we will seek to understand the functioning and chemistry of GC systems and how to adapt that function and methodology to our own analyses. We will take a deeper look into the chemistry, physics, and methodology of each piece of the GC system as we examine the different components of it (form and function) to see what changes can be made to increase resolution, efficiency, and produce better analyses.
In the last column, we looked at the first phase of the gas chromatography (GC) system, the mobile phase (1). The mobile phase for gas chromatography by its very name is a gas, whose choice and quality can change the chromatography of the system. In this second installment, we will continue to examine the flow path of the GC system from the point which samples enter the system—the inlet and the flow controller.
Finishing Gases with Flow Controllers
The transition point for gases and the inlet is the flow controller which are the values, regulators, and controllers that facilitate the movement of gases and samples together to the column. Modern GC instruments have several ways to control gas flow into the system, but the basic two modes are to control for constant pressure (Pa), and constant flow (v/s).
Carrier gas increases in viscosity as the temperature of the GC system rises during a method. The gas wants to expand with the increase of temperature and the gas at the beginning of the column will want to force its way back out the head of the column as temperatures rise. Gas near the center of column will slow as the kinetic energy of the gas increases as it heats and interacts with the column.
The initial GC systems were default constant pressure mode where the force was applied at the start of the column. For constant pressure mode, the pressure at the head of the column (head pressure) is fixed and as the sample moves through the column there is less force, and the sample can slow down. This type of mode can allow for peak broadening in later eluting peaks. A constant flow mode maintains a constant flow rate throughout the length of the column as temperature increases. Of the two techniques, constant pressure is simpler with less adjustments to the injection port where a constant flow requires a more sophisticated system with more user control of the flow. Another disadvantage is the amount of pressure generated by the constant flow mode, especially at the end of the higher temperature ramp. Systems need to be able to accommodate the resulting pressures and capacity, especially when sample and solvent enter the equation.
Starting with Sample Introduction
Most GC analyses target volatile organic compounds (VOCs). Some sources group all volatile organic compounds that have high vapor pressures and boiling points less than 250 °C as VOC or VOs. Other sources, such as the World Health Organization (WHO), divide the volatile compounds into very volatile compounds (VVOC) with the lowest boiling points, volatile organic compounds (VOC) with mid-range boiling points, and semi-volatile compounds (SVOC) with the higher range of boiling points. (See Table I.)

The most common sample introduction technique uses an analytical syringe (usually composed of glass with a needle) that withdraws up to several microliters of sample and injects it into the inlet or column. The syringe is either used to manually introduce the sample or is attached to an autosampler which loads and injects samples from a tray or rack of sample vials.
There are other types of sample introduction that employ different physical and chemical properties to deliver the sample to the GC inlet depending on the nature of the sample and analytical targets and the type of chromatography column being used. For the purpose of this article, we will focus on the most commonly used type of modern GC column called a capillary column. Capillary columns are small diameter tube-like columns where the stationary phase is contained in a film that coats the interior surfaces of the capillary column as opposed to older style packed columns which are packed with stationary phases.
Sample introduction systems are designed to maximize the chemical and physical properties of the sample and the target analytes when direct injection by syringe is not feasible for the sample form or matrix. Samples that are amenable to direct injection are in a volatile matrix such as a volatile solvent or gas and are able to vaporize in the injector. The target themselves must be able to enter the column without evaporating before they reach the head of the column or become coated in the injector where the sample will not reach the column. When samples are in complex matrices or less/more volatile than most commonly used GC solvents, then other techniques such headspace, thermal desorption, pyrolysis, or purge and trap become necessary.
Headspace sampling (HS) is an autosampler based sample introduction system primary used to determine highly volatile compounds in liquids or solids. Samples are placed in a temperature controlled and sealed vial which allows the volatile compounds to be vaporized into the open headspace of the sample vial from which the injected sample is taken. This technique is used for the analysis of very volatile compounds such as fragrances, terpenes, volatile pollutants, and solvents.
Thermal desorption (TD) is a technique where compounds are collected on a solid sorbent material to preconcentrate the sample, then quickly vaporized off the sorbent into the inlet. TD is used in environmental testing and the monitoring of hazardous gases and can be used in conjunction with other sample introduction techniques such as headspace or pyrolysis. A related technique is solid phase microextraction (SPME) where a coated fiber sorbent is introduced into a sample to capture and concentrate targeted analytes and then is inserted into the injection port for thermal desorption.
Purge and Trap (PT) sampling is most often used with highly aqueous samples. PT is a combination of several sampling techniques including headspace and thermal desorption. Volatile compounds are purged from a water-based sample into a dynamic headspace then are trapped on a sorbent material. Finally, the sample is rapidly heated to discharge the sample into the GC. This technique is used for a variety of water-based samples including environmental pollutants, food, and beverages.
Pyrolysis is used to produce volatile species from a nonvolatile sample. Samples are heated to extreme temperatures from 500 °C to 1000 °C to create volatile fragments to be analyzed by GC. This technique is mostly used for complex matrices such as geological samples, polymers, solid, and biological samples.
The choice of sample introduction is guided by the type of sample you are analyzing and the level of quantitation you need to be able to produce. (See Table II.)
Entering the Inlet
The inlet is part of the sample introduction system that is most often the first place the sample enters the GC system via the injection port. The injector or injection port is mounted to the inlet through a liner and chamber leading to the head of the GC column and introduces the sample and carrier gas to the stationary phase located inside the column. The purpose of the inlet is to concentrate or focus the analytes of interest into a band to produce sharp peaks aiding in good resolution.
There are many configurations of sample injection ports and inlets depending upon sampling technique, column type, or nature of the analytical sample. These configurations fall into categories based on temperature and introduction to the column. Flash vaporization has sample injected into a heated zone before the vapor passes to the column. On-column introduction (also known in some cases as cool-on-column) injects a liquid sample at the head of the column, either directly or into a space or void at temperatures below the boiling point of the solvent matrix. This technique is used for VVOC and VOC analyses and can reduce the risk of sample decomposition in the injector but can also be the source of column contamination or possible overload.
The most common type of sample inlet for capillary systems uses flash vaporization of the sample in a heated zone of the inlet in a split/splitless (S/SL) configuration. The liquid sample is injected into the liner located in the heated zone of the inlet. The heat volatilizes the sample and matrix, and the sample is either pulled entirely into the column by the carrier gas (splitless) or portioned in a specified ratio with a portion going to the column and the rest directed to the split valve (split). The use of S/SL modes depend on the type and concentration of the analytes in the sample. Samples with high concentrations (high ppm or %) often are run using split mode where lower concentration samples (low ppm or ppb) are more often run splitless. Even in the splitless mode, the split value can be opened for a period of time to purge interfering solvents or other interfering compounds.
Split mode is used for highly concentrated samples or samples with dirty matrices. Typical split ratios range from 1:5 to 1:500 and typical split flows range from 15 to 150 mL/min depending on the column inner diameter (ID).(See Table III.)

There are a number of potential considerations when using split mode. First there is inlet discrimination, where less volatile components do not rapidly vaporize. Once the injection enters the inlet there is a higher proportion initially of volatile compounds (even higher than the original sample), as time of the sample in the injector increases, the discrimination decreases but the band of material broadens before entering the column. Another potential issue is called backflash, when the vaporized sample expands quickly and exceeds the volume of the sample. This expansion can cause sample loss or contact with active sites or cold spots in the injector and promote condensation or ghost peaks in the analysis or subsequent samples. Reduction of sample size, increase in septum purge, or increase in liner size can help control backflash.
Splitless injections have the entire sample flow through the injector. Some of the considerations in splitless injections include solvent effects which broaden peaks. The sample can be refocused by solvent refocusing andcold trapping. The starting column temperatures are lowered to temperatures from about 10 °C below the boiling point of the sample solvent. The cooler temperature of the column condenses the higher boiling compounds and allows the sample vapor to condense at the head of the column to be trapped and refocus and flood the early zones of the column lower boiling compounds. Table IV shows typical staring oven temperatures for common GC solvents.

Programmed temperature vaporization (PTV) is a programmable valve system that many modern GC instrument offer, which allows the user to combine the inlet types to optimize their sample injection conditions. By optimizing the inlet variables of temperature, pressure, and split, they are able to maximize their GC analyses for their target analytes.
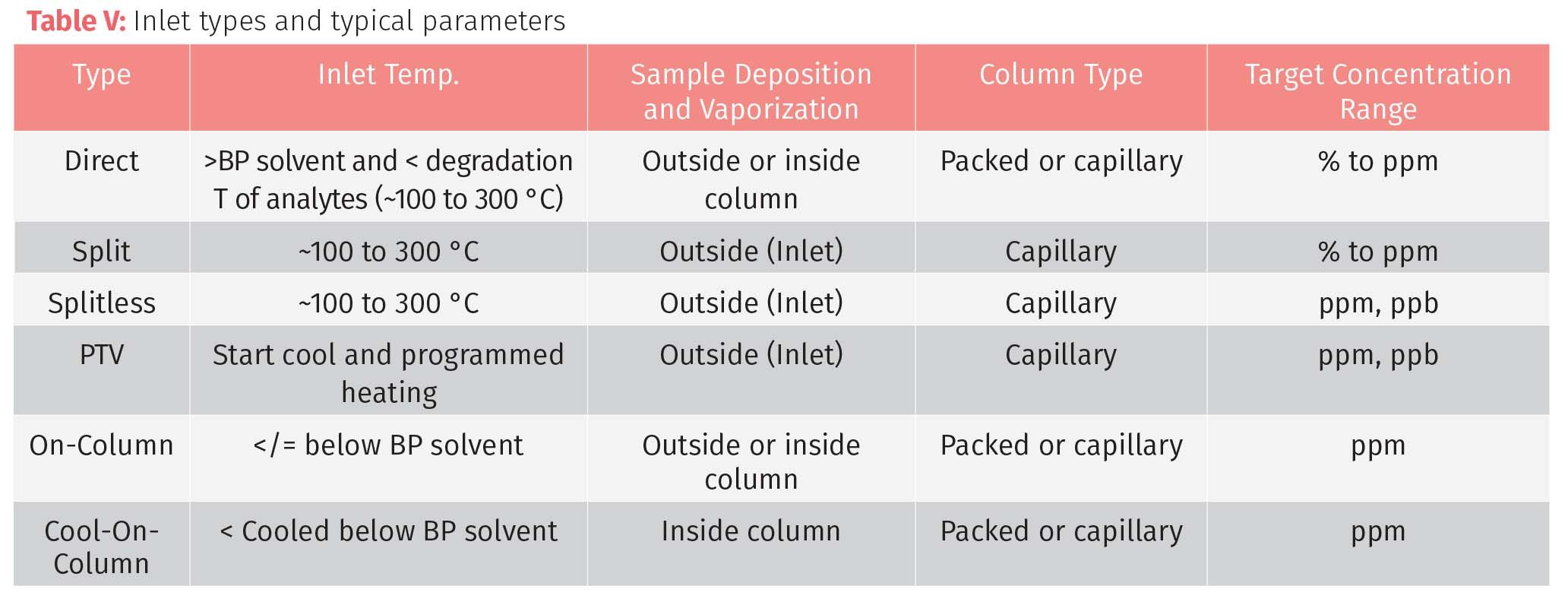
The choice of sampling technique and inlet configuration depends on the type of samples, target analytes, and column selected (see Figure 1 and Table V).

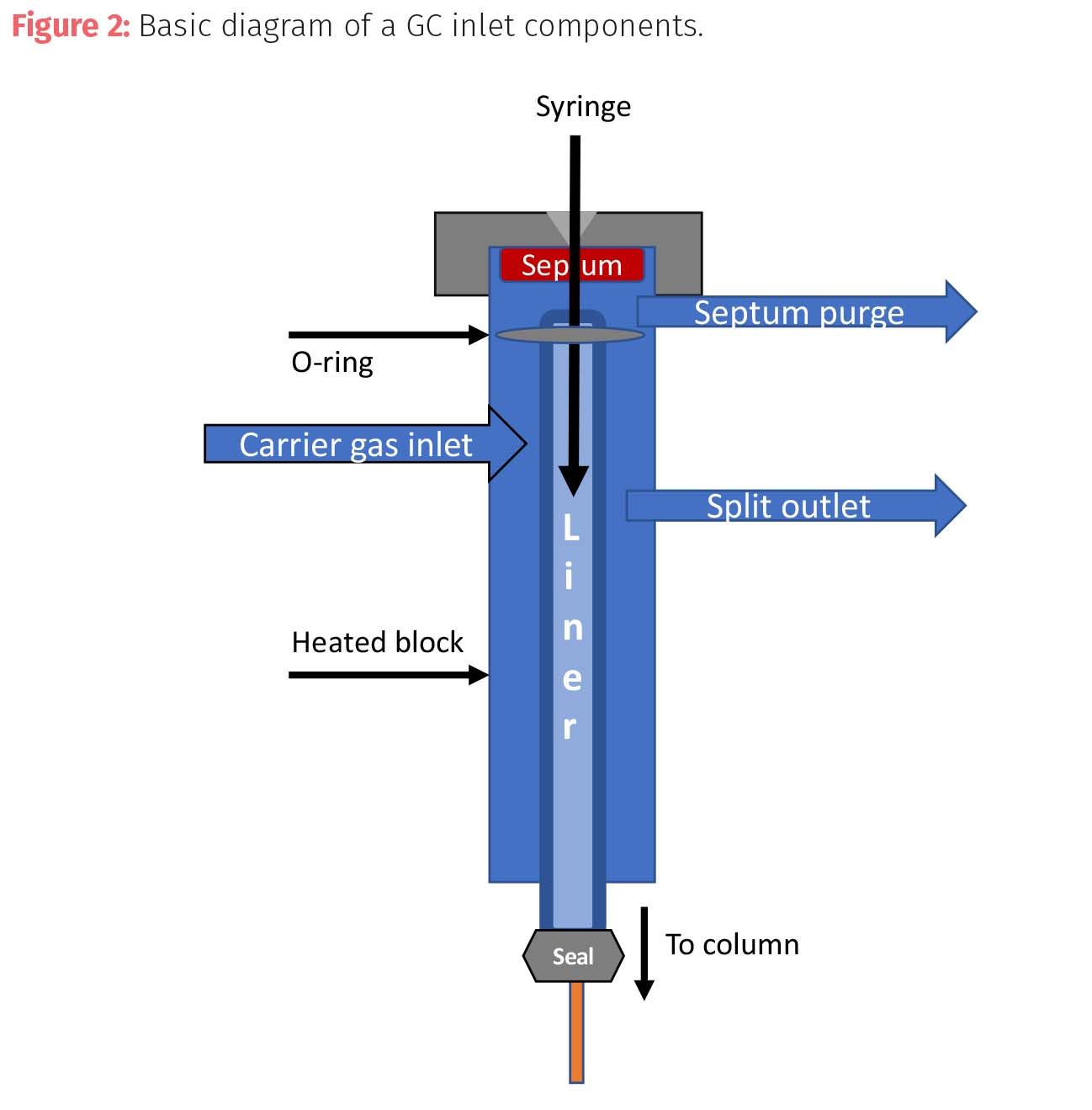
The basic components of a capillary GC inlet (Figure 2) are:
- Septum nut: a hardware nut with a small opening through which the sample syringe needle is threaded to pierce an underlying septum.
- Septum: a disc of flexible material such as silicone, rubber, PTFE, or another polymer that is relatively inert and can withstand high temperatures. The flexibility of the material allows the septum to reseal the puncture of the needle and stop the depressurization of the inlet or the interruption of the carrier gas flow. All septa have a level of off-gassing or bleed of volatile materials which can appear as sample contaminants. Critical applications should use low bleed septa or septa made of cleaner polymers such as PTFE. In addition, septa should be conditioned prior to use in an analysis by heating to analytical temperatures. As septa are used, the needle begins to core the injection site and the septa loses its ability to retain a seal which can lead to broad or tailing peaks or changes in retention time. The cored material can also fall into the liner of the inlet and cause active sites that retain samples in the liner rather than move to the column. The septa material must be able to accommodate the temperatures of the injection port (usually over 200 °C) or else the material will break down quickly and cause contamination or poor results.
- Septum purge: a purge gas (usually the carrier gas) that flows away (3–4 mL/min) from the septum to remove off gassing compounds (bleed) from the septum and any residual leakage from around the septum seal.
- Carrier gas inlet: the entrance for the carrier gas which may be connected to additional flow controllers to moderate, switch, or mix flows of carrier gases during a method.
- Heated chamber or block: method to heat the injection port area to facilitate vaporization of the sample.
- O-rings and inlet seals: sealing rings and discs made from typically inert materials to keep a seal on sections of the injection port. The bottom seal where the liner and the column meet are typically gold-plated, which can be damaged or eroded by continuous exposure to solvents or harsh analytes. The lifetime of a gold inlet seal can be extended by proper choice of a liner to limit sample contact.
- Split vent: a vent controller that allows the sample volume to either go completely to the inlet or splits the sample volume at different ratios.
- Liner: a glass or quartz reaction vessel in which the sample is vaporized. Liners can be simple tubes of glass or be more complex with internal features and architecture that promote or reduce volatilization of sample components.
Deeper Look at Liners
Liners are the reaction chamber of the inlet. The purpose of the liner is to aid in the efficient vaporization of the target analytes. The selection of a liner, both in structure and material, is dependent upon the analytical targets and their sensitivity of the analyses. Liners should ideally be free of active sites that may produce interactions between the sample and the liner material. The interaction with the active sites can cause loss of sample, unintended chemical reactions, or reduction of sensitivity while increasing baseline noise. GC analyses in the percent or high ppm range are affected less by the active sites than trace analyses which benefit from deactivated liners.
Liners can either be purchased deactivated or be deactivated by a process such as silanization. Silanization covers the active silanol sites found in borosilicate glass. The use of quartz liners and quartz glass wool can reduce the active sites. Users who choose to purchase borosilicate glass liners and glass wool can deactivate the glass with a silanization process. The basic steps of silanization include rinsing the glass with some solvent or acid then soaking the liners in a silanization agent after which the liners are rinsed, dried, and heated to bake off excess materials. A typical method would be a 5–10% solution of dimethyl disulfide (DMDS) in toluene in which the liners are soaked up to an hour or so then followed by rinsing with a solvent such as methanol and conditioning at over 200 °C for about an hour. Liners can be conditioned inside the GC oven inside a beaker if an appropriate laboratory oven is not available.
Inlet liners are available for almost any GC application or analyte; but there are some common forms that are applicable to many split/splitless configurations (Figure 3). The diameter of the liners and their geometry are dictated by either the split/splitless injection, amount of potential contamination, or form of the sample. Larger diameter liners are intended for split samples and fast injection speeds while splitless injections use more narrow liners and slower injection speeds.

Straight liners have low surface area and that benefits the user with low activity. They are easy to use and the most applicable for a wide range of VOCs. But, without any inserts, baffles, or barriers the contamination of multiple injections quickly causes them to become contaminated and can risk the longevity of the GC column if they are not changed frequently. Out of all the choices the straight liners are the cheapest option and can sometimes be reconditioned for reuse once cleaned. The addition of quartz or glass wool into a straight liner increases surface area and potentially active sites, but also traps non-volatile compounds. The disadvantage of using a straight liner with glass wool is that the wool can migrate around the liner and cause blockages.
The next type of liner is a tapered liner that has a baffle at either the top or bottom of the liner. Liners tapered to the top of the injector can reduce solvent expansion and be helpful in applications with high aqueous matrices, but aligning the taper at the top means it can potentially break more quickly or cause needle breakage. Aligning the taper at the bottom is good for peak shape and sensitivity especially with compounds such as pesticides. Double tapered liners have a baffle or taper at each end which helps retain the vapor cloud.
Spiral or cyclo liners like the taper and the use of glass wool allow for more surface area and better sensitivity, but often at the cost of price and versatility. All the features of the liners discussed can be found in a variety of combinations such as double tapered cyclo liner with or without glass wool. Each level of geometry or addition of glass or quartz wool offers a level of protection against contamination to the column and possible increase in sensitivity, but often with the additional cost for replacement and inability to reuse or recycle the liner. During method development it is often best to start with a straight or single tapered liner. If the samples are very complex or high in compounds that could contaminate the GC, then use deactivated glass wool. If the target analytes or analysis require higher sensitivity, then switch from glass to quartz wool and liners. Finally, move towards the complex liner geometries if other approaches fail to provide the needed results.
Final Thoughts
Many analysts think the most important choice for a gas chromatography system is the column, but while the column does much of the work to separate analytes and produce peaks, the initial settings and sampling/inlet systems are critical for the sample to get to the column in order to be separated. Theses choices can determine if the target analyte even reaches the column to be analyzed. Many factors need to be considered such as the form of the sample (gas, liquid, and solid), the chemistry of the target analytes (volatility and boiling point), and the concentration of the targets (ppb, ppm, or percent). The system then needs to be optimized as to flow control type, flow rate, injection volume, inlet type and parameters (split/splitless, purge, and so on). By understanding the use, mode, and effect of each of the choices, the chromatographer can optimize their sample introduction and injector to make sure their targets reach the column for analysis.
Further Reading
- P. Atknis, Cannabis Science and Technology 4(9), 12–19 (2021).
- Modern Practice of Gas Chromatography, 2nd ed., R.L. Grob, Ed. (Wiley, New York, New York, 1985).
- D. Harvey, "Contributions to Band Broadening in Chromatography | Image and Video Exchange Forum, 2013.
- W. Jennings, E. Mittlefehldt, and P.P. Stremple, Analytical Gas Chromatography, 2nd ed. (Academic Press, San Diego, California, 1997).
- D. Kealey and P.J. Haines, Analytical Chemistry; The instant notes chemistry series; BIOS: Oxford, 2002.
- H.M. McNair, J.M. Miller, and N.H. Snow, Basic Gas Chromatography, Third edition (2019 edition) (Wiley: Hoboken, New Jersey, 2019).
- Gas Chromatography, https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Instrumental_Analysis/Chromatography/Gas_Chromatography (accessed 2021 -11 -15).
About the Columnist

Patricia Atkins is a Senior Applications Scientist with Spex, an Antylia Scientific company and has been a member of many cannabis advisory committees and working groups for cannabis including NACRW, AOAC and ASTM.
How to Cite this Article
P. Atkins, Cannabis Science and Technology 5(1), 20-26 (2022).

Insights on Cannabis Testing Challenges and Industry Standards: An Interview with Douglas Duncan
August 9th 2024In light of recent headlines concerning cannabis laboratories throughout the country, Cannabis Science and Technology reached out to Douglas Duncan, Laboratory Director of Kairos Labs in Detroit, MI and member of our Editorial Advisory Board for more information. In this interview, Duncan shares his perspectives on lab shopping, major challenges in the industry today, and innovations in cannabis testing laboratories for the future. He also shares insights into consumer practices and the potential effects of a federal rescheduling of cannabis.
Measuring Microbiology, Part III: Techniques for Testing for Microbiological Pathogens
May 9th 2024In the latest installment of this series, Atkins examines techniques utilized for testing microbiological pathogens, along with the role and mechanisms of cell culture techniques including its strong points and limitations for testing cannabis products.