Looking with Light: Understanding Gas Chromatography, Part IV: Detectors
Let’s investigate the differences between the commercially available detector in terms of sample type, size, and selectivity to understand which detectors fit into the most common types of cannabis GC analyses.
In previous columns, we have looked at all the components necessary to achieve chromatographic separation in gas chromatography (GC) systems. Now, we will look at the final critical component to achieving successful chromatograms: the detector. The first components of the GC system are most critical for the separation of individual sample components, but it is the detector that actually specifies which types of components will be measured to create the final chromatographic results. Detectors range in functionality from simple general-purpose detectors (nonselective) to more advanced and sensitive (selective) detectors. In this column, we will investigate the differences between the commercially available detector in terms of sample type, size, and selectivity to understand which detectors fit into the most common types of cannabis GC analyses.
The components of a chromatographic system can be likened to instruments in an orchestra and the method the piece of music.Some instruments are almost truly essential like percussion while others expand the music or shape the direction of the sound. Like an orchestra, if the system is not tuned even the best method can produce jarring results. If the method is not well written, the composition can fall flat even with the best orchestra.
The final piece or the detector is the place where all this music or data comes to life. Imagine the difference between listening to a piece of music played in a small concert space versus an open area venue. Even though the instruments, musicians, and music are the same, the notes and flavors of the music will be different; losing some parts and being overwhelmed by others. The detector in a chromatographic system is that final filter which produces that final product of all the work in selecting mobile phases, stationary phases, instruments, columns, and settings. The detector, if chosen poorly is like listening to a concert on a poorly tuned radio compared to being at the event live.
Classifications of Detectors
The choice of detector is determined by many factors including the types of analytes, the concentration of the targets, and the fate of the sample being tested. Gas chromatography detectors are grouped under several categories.First, detectors can be either selective or nonselective (universal). Selective detectors are by their name, selective as to the types of analytes that can be detected and appear in the chromatogram. Selective detectors include detectors that only react with halogens, specific elements (S, N, C, P), or compounds with specific functionality (electronegativity). Nonselective or universal detectorswork with a larger range of detectable compounds and are not limited to specific types of compounds or groups of elements (Table I).
Another way to look at detectors is by the fate of the sample after detection. Systems that consume or destroy the sample by evaporation, combustion, or mixing with other reagents to then measure the resulting materials are destructive detectors. Detectors that allow for recovery of the effluent or isolation of analytes are non-destructive detectors (Table I).
A final classification of for gas chromatography detectors is their type of response as a function of either their measurement of concentration or mass. Concentration detectors measure an analyte’s concentration in the mobile phase whereas mass flowormass detectors measure the absolute amount of the analytes in the carrier gas (Table I).
Understanding Universal Detectors
The choice of a detector is often heavily driven by the types of samples and analytes that the laboratory will be analyzing. Many laboratories depend heavily on universal or nonselective detectors to be the workhorse for the laboratory and hope that the wide range of functionality will suit most applications. The three most common universal detectors are the flame ionization detector (FID), the thermal conductivity detector (TCD), and the mass spectrometer (MS).
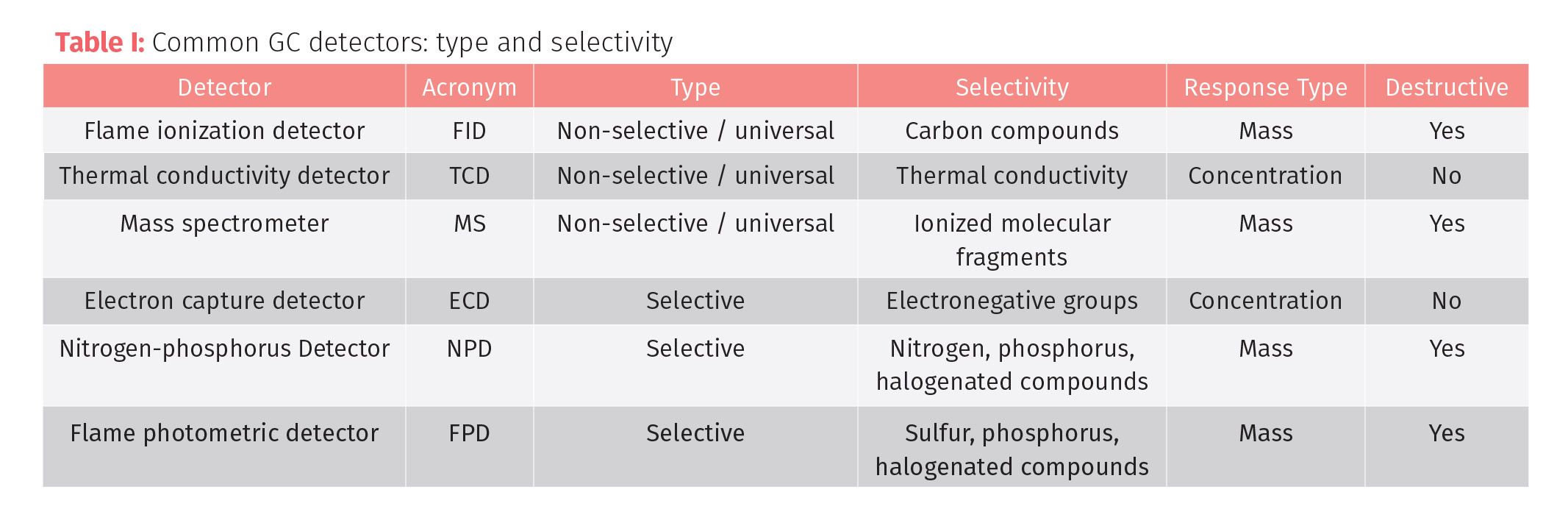
The most popularly used universal detector is the flame ionization detector or FID. It is sensitive to a wide variety of compounds with the exception of most inorganic gases, noble gases, halogenated compounds, and a few others. The FID is selective to hydrocarbons. It does not detect common contamination or background chemicals like carbon dioxide and water, which increases sensitivity to hydrocarbons. The FID is a destructive mass detector that measures the ions formed during combustion of the analytes in an air-hydrogen flame. In the flame, the sample undergoes pyrolysis to produce ions.
The ions produced are proportional to the concentration of the analytes in the gas phase. The ions are detected by the ion potential difference from two oppositely charged electrodes. The positive electrode is the nozzle where the flame is produced and where the negative electrode is a collector plate where the ions collect and produce a current upon colliding with the plate, which is measured as number of carbon atoms per unit of time and interpreted by an integrator into the chromatogram. Most responses are measured as time (x-axis) versus ion response (y-axis). This measurement is not affected by gas flow rate changes and is dependent on the mass of carbon atoms detected in a unit of time making it a mass detector (Figure 1). The advantages of the FID are its ease of use, sensitivity, wide range of analytes, resistance to interference from gases and water, and since it is a commonly used detector, the costs are less expensive than some other types of detectors. The disadvantage of using FID is that some compounds without a carbon-hydrogen bond are difficult to detect and the samples are destroyed during combustion.
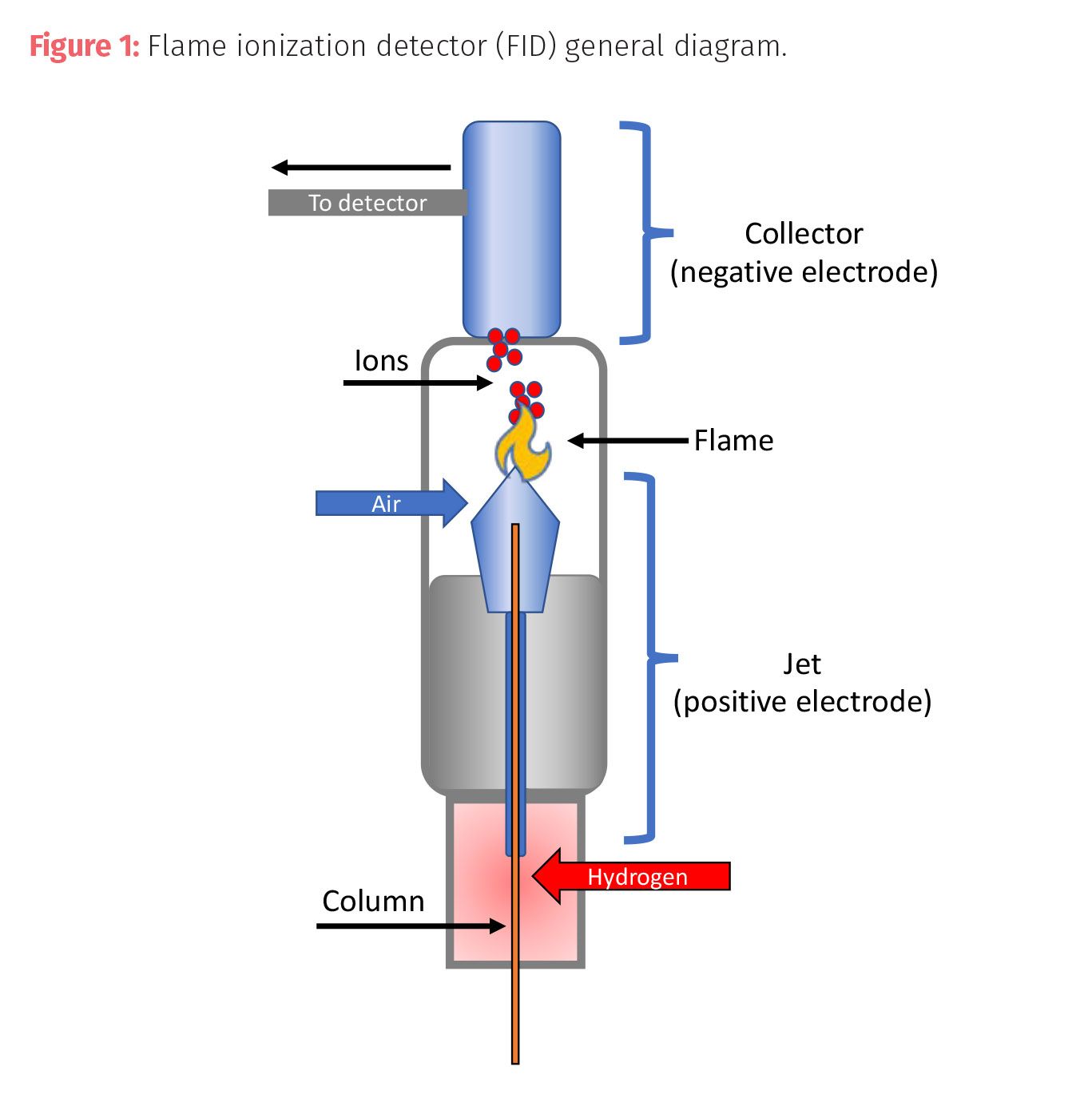
The second type of universal detector is the thermal conductivity detector (TCD) or a katharometer.The TCD is a nondestructive concentration detector that measures the change in the thermal conductivity of the carrier gas by the presence of an analyte. The TCD contains a heated filament in a source made from a thin platinum, gold, or tungsten-rhenium wire whose power is kept constant by an applied current. The temperature of the source is dependent on the thermal conductivity of the gases. The resistance in the wire is dependent on temperature, which in turn is dependent on the thermal conductivity of the gas. As compounds elute from the column, they mix with the carrier gas and the conductivity decreases, which increases the filament temperature and resistance which then changes the current causing a response in the detector. The sensitivity is proportional to the filament current and inversely proportion of the incurred temperature and the flow rate of the gas. This makes the detection a measurement of concentration.
TCDs usually contain two detectors: one is the reference for the carrier gas and the other measures the conductivity of the sample and carrier gas mixture. Gases, such as helium or nitrogen, typically used in this application have a high thermal conductivity which increases the sensitivity of the sample detection. The advantages for a TCD are ease of use and a wide range of analytes, but the disadvantages include lower sensitivity than an FID which is two to three times more sensitive than TCD. TCD is able to see gases not visible to an FID detector, but it is also subject to more background noise from common contamination like water and carbon dioxide.
The third most commonly used type of universal detector is the mass spectrometer (MS), which we discussed in a previous column (1). As the name suggests, the MS is a mass detector rather than a concentration detector where compounds are subjected to ionization and the mass-to-charge ratio of charged particles results in a mass spectrum (intensity versus mass-to-charge plot). The ions can also be measured and plotted by abundance (y-axis) versus time (x-axis) to create a total ion chromatogram (TIC). The TIC is necessary to calculate quantitative results where the spectrum provides information on compound identity. Spectra for gas chromatography–mass spectrometry (GC–MS) are well documented and characterized by many organizations including National Institute of Standards and Technology (NIST) and these spectrum are widely available as either free or paid databases often incorporated into a manufacturer’s MS system.
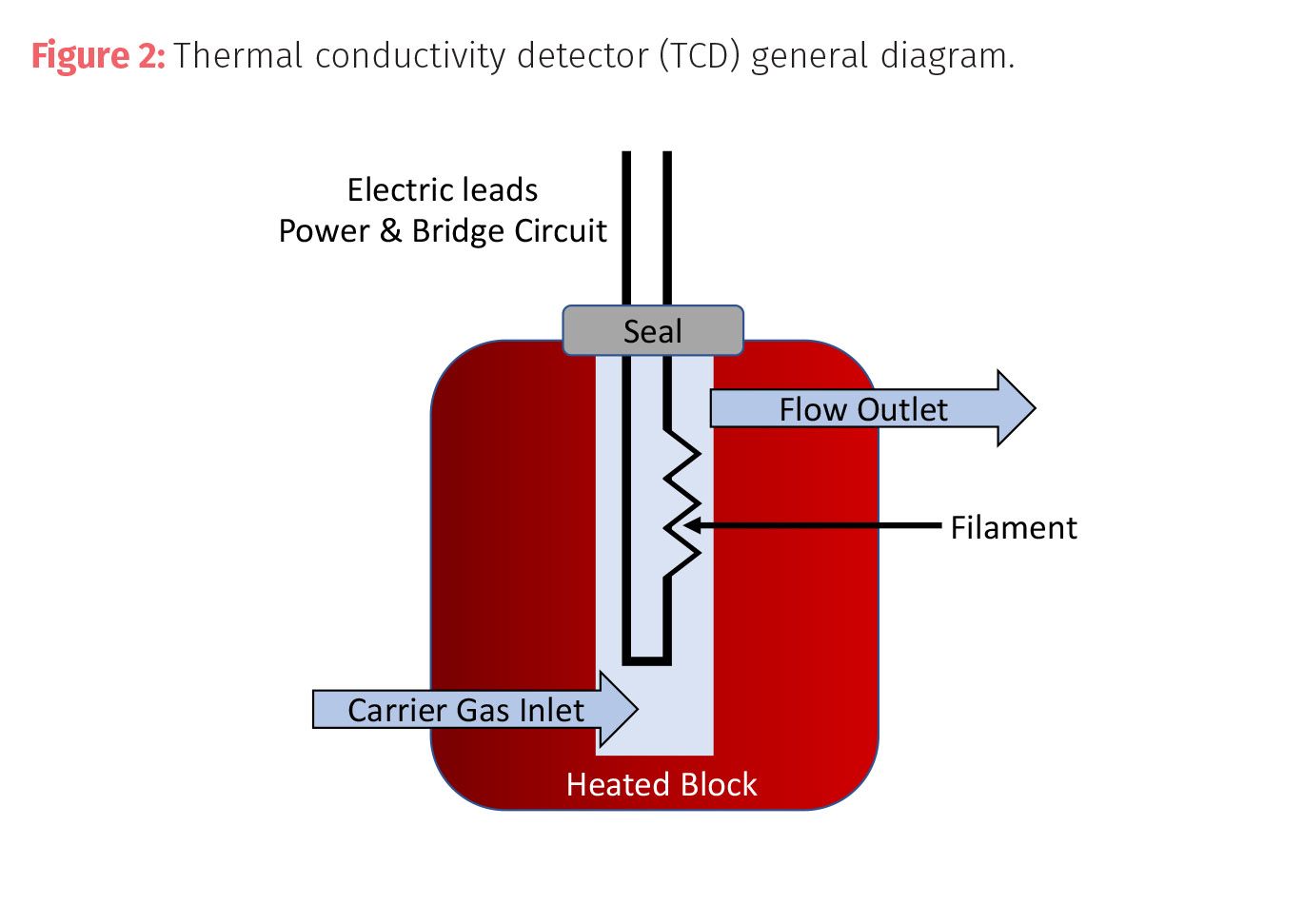
The main parts of a mass spectrometer are an inlet or transfer line, an ion source, a mass analyzer, and a detector (Figure 3). Sample inlets allow for the controlled introduction of a gaseous or vaporized liquid sample (or solid via a heated probe) through an aperture where the sample passes to an ionization source that generates ions. Most ionization techniques fall into either “hard” ionization or “soft” ionization depending on the ionization energy involved and the degree of fragmentation that results.
Hard ionization uses high quantities of energy in fragmenting the target molecules and result in a large number of fragments from the rending of bonds in the original molecule.The fragments tend to have lower mass-to-charge ratios (m/z) than the parent molecule. The most common hard ionization technique for organic molecules is electron impact ionization (EI) that uses a high-energy electron beam (~70 eV) to form radical cations which then decompose to smaller fragments. These fragments are the basis for the mass spectrum (sometimes referred to as the molecular fingerprint) and its use in identifying compounds (Figure 4). EI requires a system that can be kept under high vacuum.
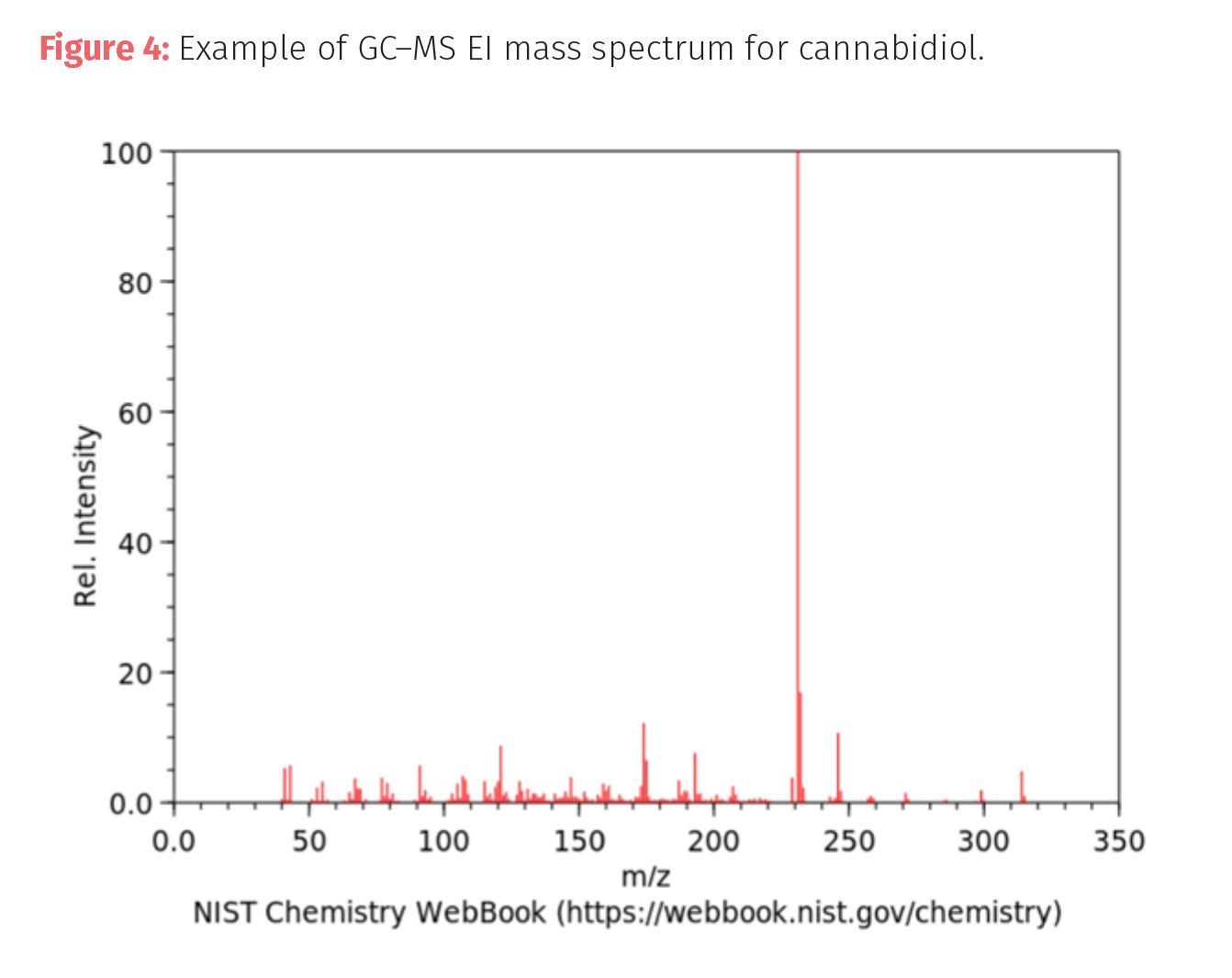
Soft ionization uses small amounts of energy to ionize molecules and result in only a small number of fragments. The most commonly used soft ionization for GC–MS is chemical ionization (CI). In chemical ionizationtechniques, ion fragments are produced by the collision between sample molecules and a collision gas. This type of ionization requires lower energy than other types of ionization depending on the type of sample and collision gas.
CI often provides simpler spectrum with little to no fragmentation. In CI, the molecular ion peak [M+1]+ is present and is helpful to determine molecular mass. This simpler spectrum can limit the amount of structural information for a particular sample or element but can be useful when other stronger ionization techniques—such as EI—that can make molecular ion peaks undetectable. CI techniques, similar to EI techniques, tend to be used in conjunction with systems under high vacuum.
The benefit of MS detectors is that they are very sensitive and provide information regarding compound identity in addition to quantitative data. The chromatographic separation is not as important to accurate quantitation since coeluting peaks can be separated by mass in the MS. There are many types of MS systems including some MS-MS tandem systems. Some of the disadvantages to mass spectrometry systems include cost, expertise, and operation. These systems do cost more than other universal detectors and require extra components such as vacuum pumps. There is more data produced for interpretation and the learning curve for an MS system can sometimes be more difficult than the other detectors.
Staying Selective with Detectors
Selective detectors, at first glance, seem to be almost like unitaskers in a laboratory needing multitaskers. These detectors are specialists at the compounds they detect. They are not meant for every type of analysis. Reverting back to our music metaphor, they are the conga drums when a Latin beat is required or the trill of the piccolo in a rousing Sousa march to get everyone clapping. These instruments are not the go-to instruments of the laboratory (or orchestra), but when you need their skills, they are often irreplaceable.
The first detector is commonly used in environmental laboratories where there are a lot of compounds with electronegative groups that are sometimes not amenable to other detectors, such as polychlorinated biphenyls (PCBs) and organochlorine pesticides. The electron capture detector (ECD) is a selective, nondestructive concentration detector best used for trace level detection of compounds with functional groups such as halogenated compounds, conjugated double bonds, peroxides, quinones, nitriles, nitrates, and other electronegative groups.
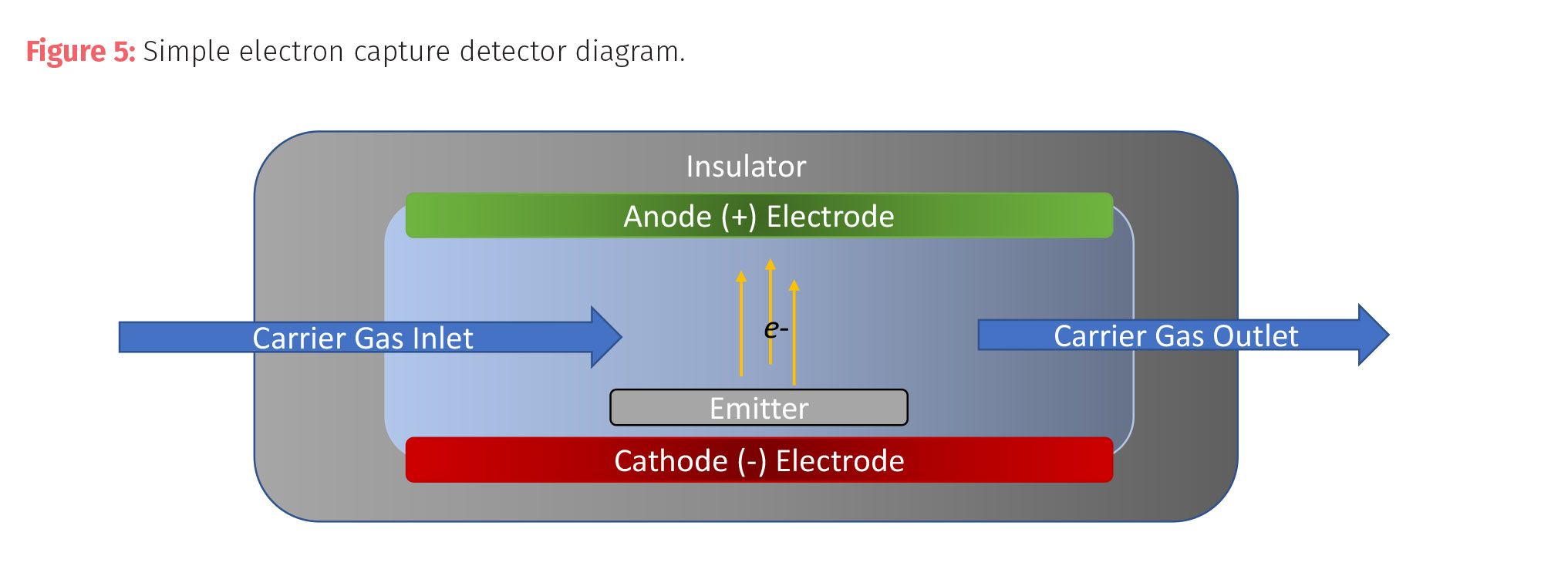
An ECD uses electrons emitted from a radioactive nickel-63 or tritium emitter. The emitter ionizes the carrier gas (commonly nitrogen) to release electrons. A constant current passes between two electrodes. Analytes pass over the emitter upon leaving the column and decrease the current between the electrodes by capturing of electrons with the analyte’s electro-negative functional groups. The detector is unable to detect changes in current for analytes that lack electro-negative groups. The loss of charge is measured, and signal is produced.
An ECD is highly selective and sensitive to analytes with electronegative functionality (up to 1000x more than FID and 106 more than TCD); however, it has issues with limited signal range and dangerous components because of radioactivity. These detectors are best suited for specific applications such as pesticide, herbicide, or PCB analysis.
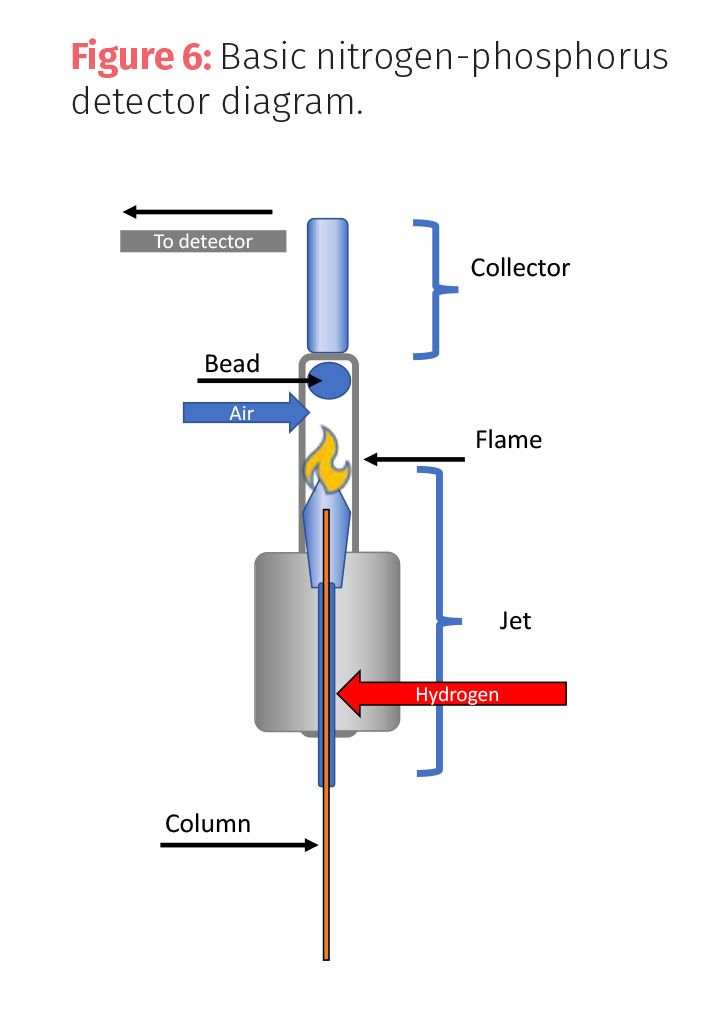
Nitrogen-phosphorus detectors (NPD) are selective, destructive mass detectors that can be used to detect analytes with nitrogen, phosphorus, or halogen substituents. NPD are often found in laboratories that test for low levels of drugs or pesticides. This detector burns compounds in a plasma around a rubidium bead (collector) with hydrogen and air. Compounds with nitrogen, phosphorus, or halogens produce ions that are attracted to the bead. The number of ions colliding with the collector is measured (Figure 6).
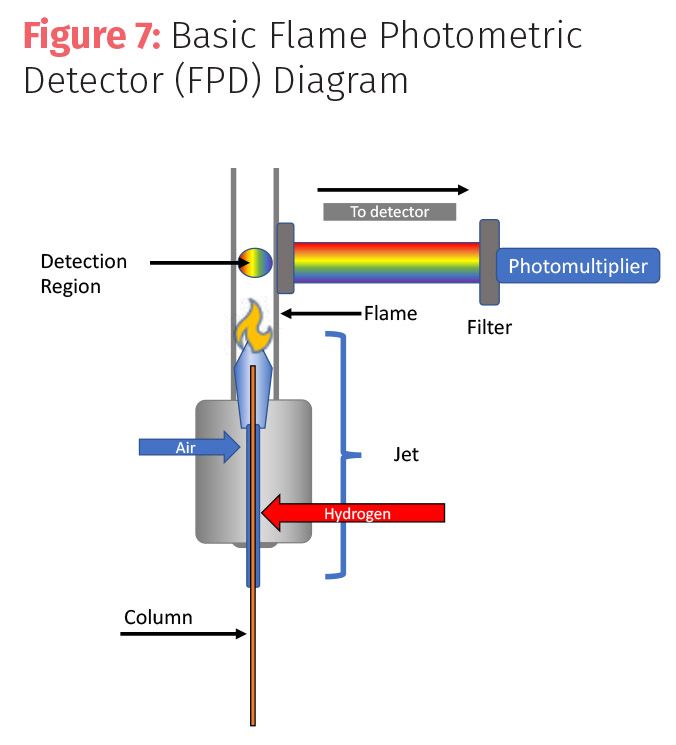
The final commonly used selective detector we will look at is the flame photometric detector (FPD). This destructive mass detector can detect halogenated and sulfur compounds like the NPD but instead of nitrogen, the FPD can also detect tin or sulfur compounds. These compound can include important pollutants such as mercaptans, sulfides, and alkyl tins found as by-products of petroleum, paper processing, and marine antifouling paints that prevent barnacle and mussel growth on oil rigs, boats, and barges.
The FPD uses a hydrogen-air flame to burn compounds to produce light. A monochromatic filter allows the selected wavelengths of light to pass to a photomultiplier to generate a signal. Each wavelength of light corresponding to a particular light producing species (that is, sulfur 394 nm or phosphorus 526 nm) requires a different filter to be detected; so, species can only be measured one at a time.
Final Thoughts
The decision of which gas chromatography detector to choose comes down to the type of analyses that you will be performing, the chemistry of the analytes, and the degree of sensitivity needed for the analysis. Universal detectors are often average or pretty good for a lot of targets but not particularly great at detecting any one target or getting down to very low detection limits. Selective detectors are great only for a small number of targets making their range of use very narrow; but for the compounds they do detect, they can measure with great sensitivity (Table II).
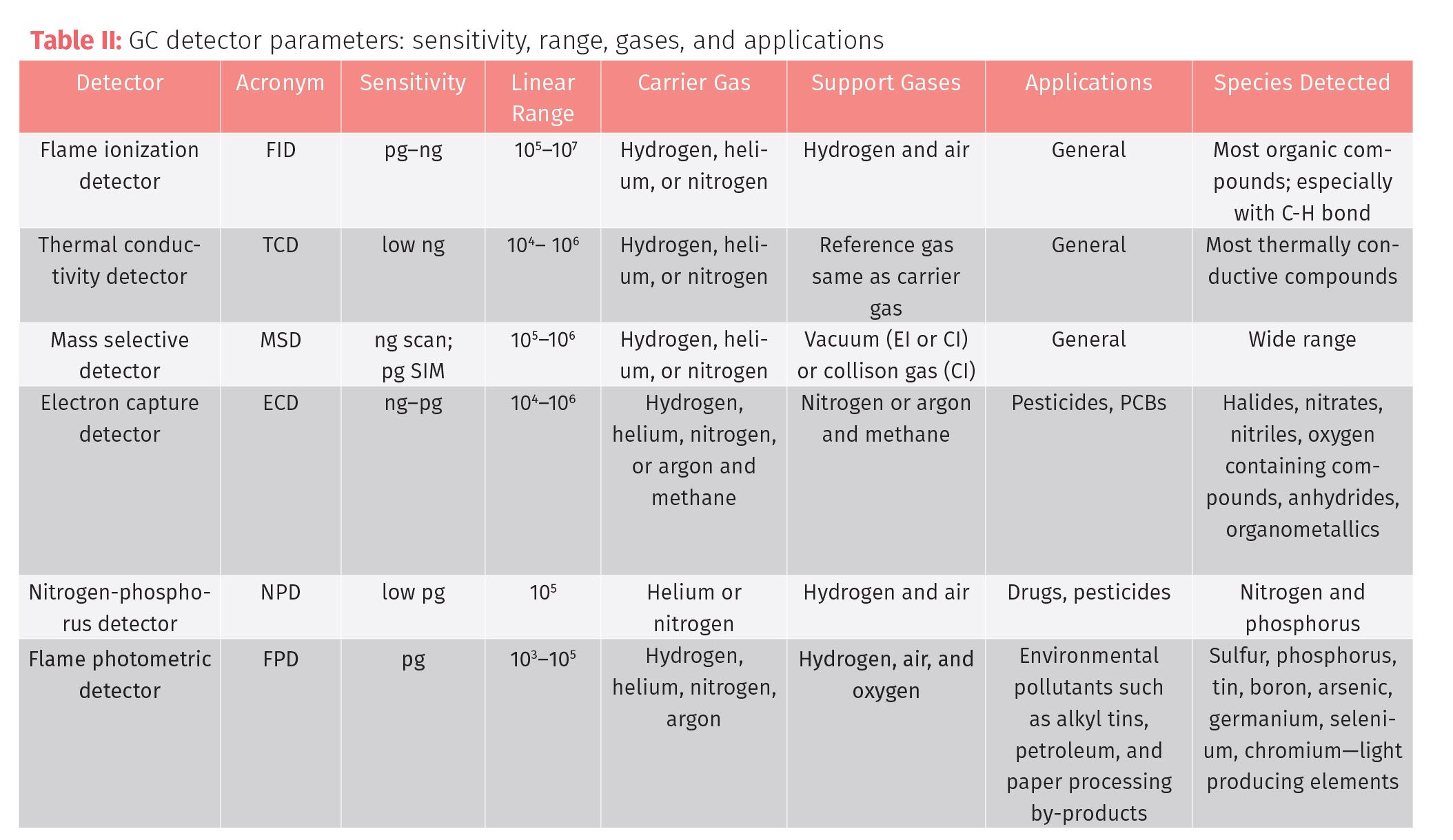
Other considerations in choosing detectors could be ease of use, amount of training required, cost of systems, and cost of additional components such as makeup gases, vacuum pumps, and so forth. The workhorses for the cannabis GC laboratory should always start with an FID and an MS system to cover both the range of concentration and compounds. If a choice has to be made between the FID system and an MS system, often the MS is a better choice since most samples can be diluted down to an analytical range on an MS, but there are many trace analyses out of the range of many FID systems.
As for the more selective GC detectors, they can have their place in the cannabis laboratory if some targets are not well detected using an FID or MS system. These detectors can be utilized to fully investigate some of the more problematic pesticides that are not well suited for FID or MS. Selective and specialized GC detectors such as an ECD or NPD can be considered to increase accuracy of selected pesticide screenings.
Hopefully this look into the different types of GC detectors has given you a deeper understanding of gas chromatography systems so that you can make knowledgeable choices that work with your samples and chemistries to produce better chromatography and accurate results.
Further Reading
- P. Atkins, Cannabis Science and Technology® 4(6), 26-38 (2021).
- Modern Practice of Gas Chromatography, 2nd Ed., R.L. Grob, Ed. (Wiley, New York, New York, 1985).
- W. Jennings, E. Mittlefehldt, and P.P. Stremple, Analytical Gas Chromatography, 2nd Ed. (Academic Press, San Diego, California, 1997).
- D. Kealey and P.J. Haines, Analytical Chemistry; The instant notes chemistry series (BIOS, Oxford, UK, 2002).
- H.M. McNair, J.M. Miller, and N.H. Snow, Basic Gas Chromatography, Third edition (2019 edition) (Wiley, Hoboken, New Jersey, 2019).
- Introduction to GC - Introduction to GC, Chromedia: https://www.chromedia.org/.chromedia?waxtrapp=xqegzCsHiemBpdmBlIEcCzB&subNav=tlpbfDsHiemBpdmBlIEcCzBsB (accessed 2022 -01 -11).

About The Columnist
Patricia Atkins is a Senior Applications Scientist with Spex, an Antylia Scientific company and has been a member of many cannabis advisory committees and working groups for cannabis including NACRW, AOAC and ASTM.
How to Cite this Article:
P. Atkins, Cannabis Science and Technology® Vol. 5(5), 18-25 (2022).

Insights on Cannabis Testing Challenges and Industry Standards: An Interview with Douglas Duncan
August 9th 2024In light of recent headlines concerning cannabis laboratories throughout the country, Cannabis Science and Technology reached out to Douglas Duncan, Laboratory Director of Kairos Labs in Detroit, MI and member of our Editorial Advisory Board for more information. In this interview, Duncan shares his perspectives on lab shopping, major challenges in the industry today, and innovations in cannabis testing laboratories for the future. He also shares insights into consumer practices and the potential effects of a federal rescheduling of cannabis.